Suspension culture method of adherent cells and application thereof
A technology of adherent cells and suspension culture, applied in the field of suspension culture of adherent cells, can solve problems such as high cost, cell apoptosis, and unsatisfactory cell density, and achieve the effect of improving production efficiency and survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
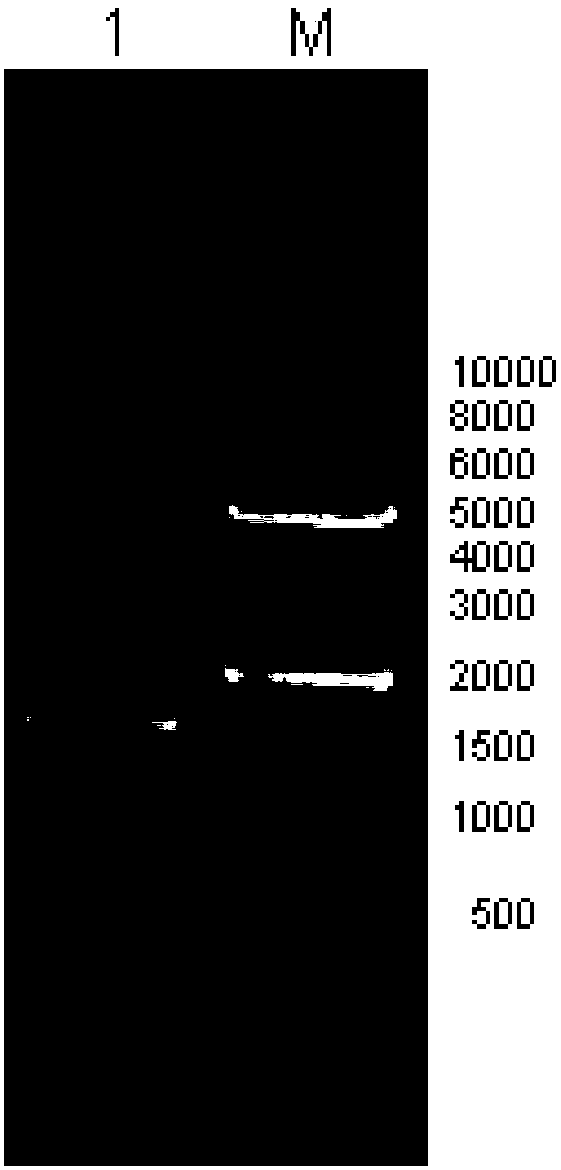
[0038] 1. Human mmp3 gene cloning:
[0039] (1) Synthetic primers are as follows:
[0040] Primer PHMMPF3: 5'-TCGACCACCATGAAGAGTCTTCCAATCCT-3';
[0041] Primer PHMMPR3: 5'-TCAACAATTAAGCCAGCTGT-3'.
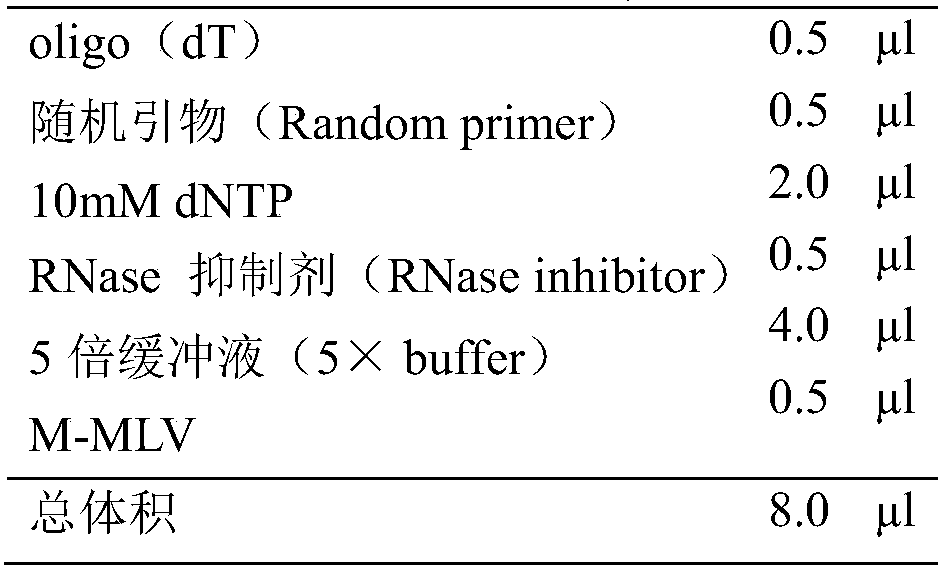
[0042] (2) Template cDNA preparation
[0043] I. Total RNA extraction
[0044] A. Add leukocyte separation solution to human peripheral blood, collect human blood leukocytes by centrifugation at 1500rpm, add 1ml Trizol (Invitrogen) solution, blow and mix well to fully lyse the cells, and let stand for 5 minutes;
[0045] B. Add 200 μl of chloroform, vigorously shake and mix for 30 seconds, make the aqueous phase and organic phase fully contact, and let stand at room temperature for 15 minutes;
[0046] C. Centrifuge at 10,000g for 15 minutes at 4°C. It can be seen that it is divided into three layers, and the RNA is in the upper aqueous phase. Move to another new RNase free EP tube;
[0047] D. Precipitate RNA: Add 0.5ml isopropanol, mix gently and fully, and let stand at room...
Embodiment 2
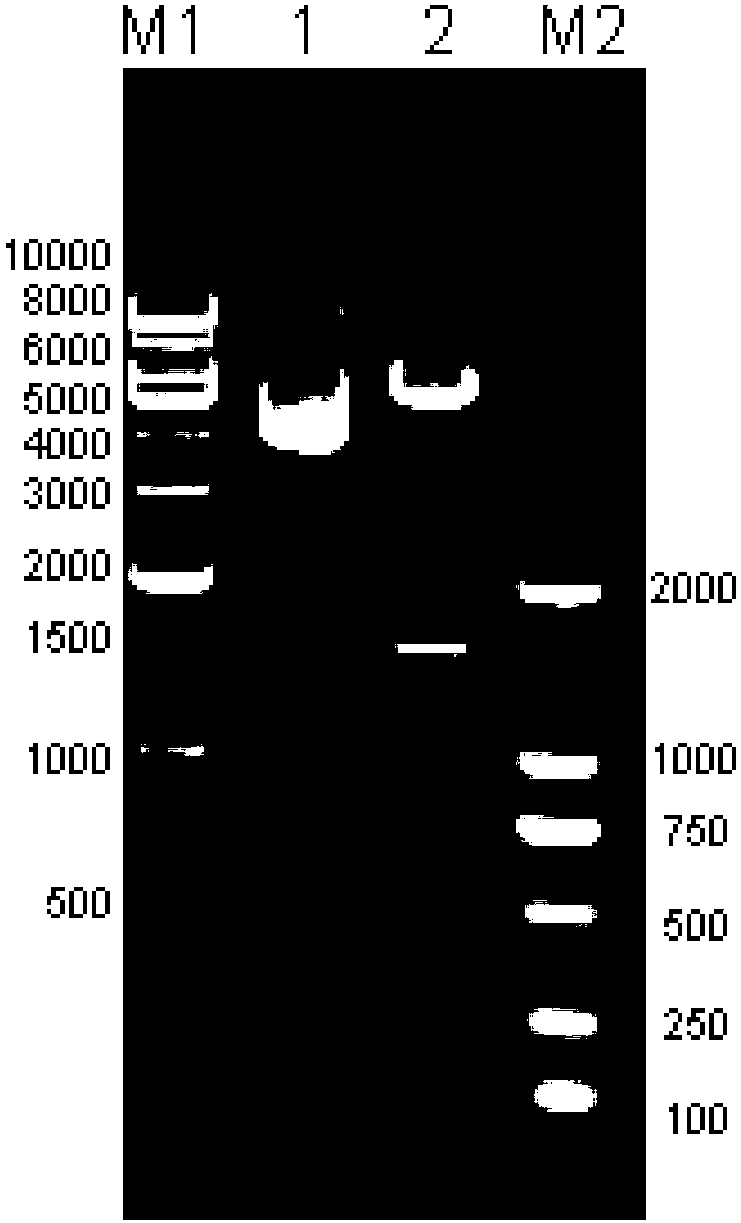
[0149] The operating steps are basically the same as in Example 1, except that the cell line used is VERO cells (ATCC-81), the human MMP10 gene is cloned, and recombined into the pcDNA3.1(+) vector.
[0150] Wherein, the primer sequences used are as follows:
[0151] Primer PHMMPF10: 5'-CTGGCTAGCCACCATGGAGCCACTGGCCATCCT-3';
[0152] Primer PHMMPR10: 5'-AGACTCGAGTCAGCAGAGGAGCCAGCTGT-3'.
[0153] The sequencing sequence of the MMP10 gene of the positive clone is as follows:
[0154]ATGGAGCCACTGGCCATCCTGGTGCTTCTGTGCCTGCCAGTCTCTTCAGCATATCCTCTGCATGGAGCAGTGAGGCAAGACCACCCAAGCATGGATCTTGCTCAGCAATATCTAGAAAAGTACTACAACTTTGAAAAAAATGAGAAACAAGTATTTAGAAGAAAGGACAGTAGTCCCATTGTCAAAAAAATCCAAGAAATGCAGAAGTTCCTCGGGCTGGAGGTGACAGGGAGGCTGGACTCCAGCACTATGGATGTGATGCTCAAGCCCAGGTGTGGCGTCCCCGATGTCGGTGGCTTCACCACCTTTCCAGGTTCACCAAAGTGGAGGGAAACAAACCTCACCTACAGGATTGTGAATTATACACCAGATTTACCGAAAGAGAGTGTGGACTCTGCTATTGAGAAAGCACTGAAAGTCTGGGAAGAGGTGACCCCACTCACATTCTCCAGGCGCTCTGAAGGAGAGGCTGACATAATGATCTCCTTCGCAGCTGGAGAACATG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viable cell rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com