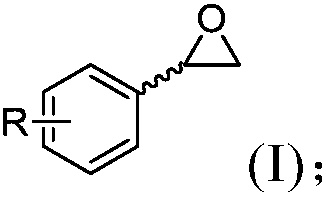
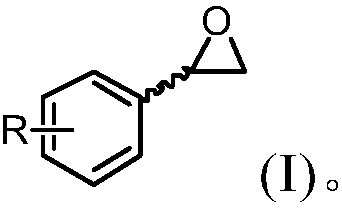
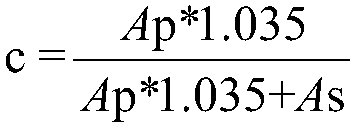Epoxide hydrolase mutant and application thereof in enantiomeric normalizing hydrolysis of epoxides
A technology of epoxy hydrolase and epoxide, which is applied in the fields of chemical synthesis and enzyme engineering, and can solve problems such as imperfect stereoselectivity, unsatisfactory optical purity of hydrolyzed product ortho-diol, and poor enzyme activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1, construction of epoxyhydrolase VrEH2 mutant
[0095] Plasmid pET28a-VrEH2 was constructed as follows: the mRNA sequence isolated from mung bean flour was used as a template, and the following primers F1 and R1 were used to amplify to obtain an amplified fragment containing the base sequence shown in the sequence table SEQ ID No.1, which was Inserted into the BamH I / Xho I site of the expression plasmid pET28a.
[0096] F1: 5'-CGCGGATCCATGGAAGAAATAGAACA-3' (SEQ ID No.3);
[0097] R1: 5'-CCGTAAAACTTCTTGATAAAATCGTA-3' (SEQ ID No.4);
[0098] The present inventors obtained the protein crystal structure of epoxyhydrolase VrEH2 by using X-ray diffraction method. On the basis of the structure, the binding mode of epoxy substrate was simulated by molecular docking, and the binding mode of substrate was predicted by POVME software. Combine pockets. Subsequently, by analyzing the substrate activity around the pocket The amino acids within the range lock candidate...
Embodiment 2
[0099] Example 2 Induced expression and purification of epoxyhydrolase VrEH2 mutant
[0100] Inoculate the recombinant expression transformant strain of the recombinant expression epoxyhydrolase VrEH2 mutant obtained in Example 1 into an LB medium test tube containing 50 μg / mL kanamycin, and culture it on a shaker at 37°C and 180rpm for 12 hour, then inoculate it into a 500mL Erlenmeyer flask containing 100mL LB medium at an inoculum size of 1% (v / v), place it in a shaker at 37°C and 180rpm, and cultivate it when the OD of the culture solution 600 When it reaches about 0.6, add IPTG to a final concentration of 0.2mmol / L to induce expression. After induction at 25°C for 12 hours, the culture solution is centrifuged at 12,000rpm for 15min to collect the bacteria, and wash the bacteria twice with 0.85% normal saline. to obtain resting cells. Weigh 0.1 g of the obtained wet cells, resuspend in 10 mL of Tris-HCl buffer (100 mM, pH 6.5), and perform the following ultrasonic treatme...
Embodiment 3
[0101] Example 3 Epoxyhydrolase VrEH2 Mutant Activity and Stereoselective Determination
[0102] The activity of the epoxyhydrolase VrEH2 mutant was determined by reversed-phase HPLC (C18 column), using p-nitrostyrene oxide as the substrate for activity determination, and the activity system included 50 μL substrate (20 mM, dissolved in DMSO), 50 μL Appropriate concentration of enzyme solution and 400 μL potassium phosphate buffer (100 mM, pH 6.5). The reaction was carried out in a 2mL EP tube at 30°C and 1000rpm shaking. After 10 minutes of reaction, 100μL was sampled, and 400μL of methanol was added to stop the reaction, centrifuged at 12,000rpm for 2min, and the centrifuged supernatant was filtered with a 0.22μm membrane, and then HPLC was performed on a C18 column For analysis, the mobile phase is methanol:water=75:25, the column temperature is 30°C, the flow rate is 0.8mL / min, and the detection wavelength is 270 / 220nm. The specific activity of the enzyme was calculated a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com