Epoxy hydrolase, gene thereof and application thereof
An epoxy hydrolase and gene technology, applied in the field of bioengineering, can solve the problems of narrow substrate spectrum, low enzyme yield, low overall catalytic activity, etc., and achieve the effects of mild reaction conditions, high catalytic efficiency and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Cloning of Epoxyhydrolase Gene
[0044] According to the B. megaterium (B. megaterium) QM B1551 hydrolase gene sequence BMQ_2920 and B. cereus (B. cereus) ATCC 10987 hydrolase gene sequence BCE_0353 included in Genbank, the PCR primers were designed as follows:
[0045] Primer 1 (amplification of BMEH I gene):
[0046] Upstream primer: CAC GGATCC ATGAGTAAACAGTATATAAACGT
[0047] Downstream primer: GGC GTC GAC TTACTTATTTAAAAAATTCCACAT
[0048] Primer 2 (amplification of BMEH II gene):
[0049] Upstream primer: CAC GGATCC ATGGAGAAAGTAAAAGCAATACT
[0050] Downstream primer: GGC GTC GAC TTACACATTAGACTTTCCTTTTTC
[0051] Wherein, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the SalI restriction site.
[0052] Using the genomic DNA of B. megaterium (B. megaterium) CGMCC No.1293 as a template, PCR amplification was performed with primer 1 and primer 2, respectively. The PCR s...
Embodiment 2
[0055] Embodiment 2 Preparation of recombinant expression vector (plasmid) and recombinant expression transformant
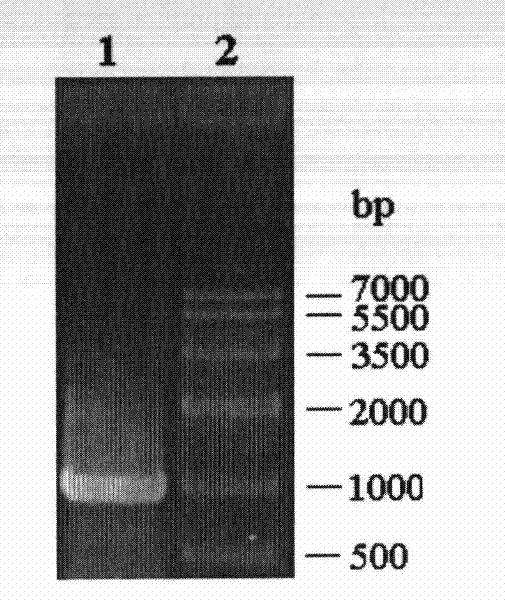
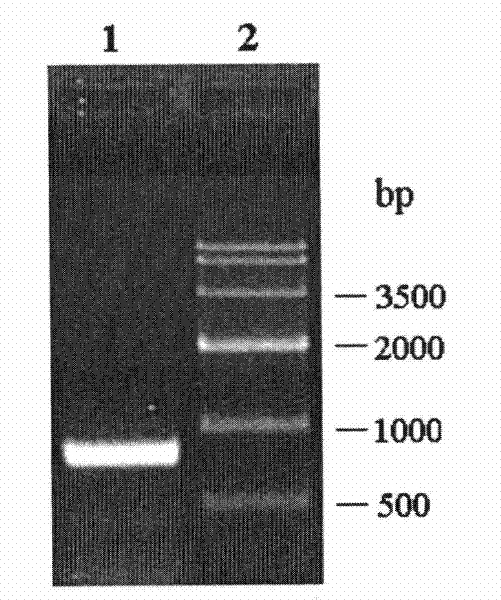
[0056]The PCR product obtained in Example 1 was ligated with the pMD-18T vector to construct cloning plasmids pBMEHI-18T and pBMEHII-18T, respectively. Then they were transformed into E.coli DH5α competent cells. Positive clones were screened by colony PCR, plasmids were extracted, digested with restriction endonucleases BamHI and SalI at 37°C for 12 hours, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit, which contained The correct insert (see respectively image 3 and Figure 4 ). The target fragment was mixed with the plasmid pET28a digested with BamHI and SalI, and ligated overnight at 4°C under the action of T4 DNA ligase to obtain recombinant expression plasmids pET-BMEHI and pET-BMEHII.
[0057] The above recombinant expression plasmids were transformed into E.coli DH5α competent cel...
Embodiment 3
[0058] Example 3 Expression of Recombinant Epoxyhydrolase
[0059] The two strains of recombinant Escherichia coli obtained in Example 2 were respectively inoculated into LB medium containing kanamycin, and cultured with shaking at 37° C. overnight. Insert the inoculum amount of 1% (v / v) into a 250ml Erlenmeyer flask equipped with 50ml LB medium (peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0), place at 37°C, 180rpm Culture on a shaker. When the OD of the culture medium 600 When reaching 0.6, add IPTG with a final concentration of 0.5mmol / L as an inducer. After induction at 16°C for 20 h, the culture medium was centrifuged to collect the cells and washed twice with saline. The resulting resting cells were suspended in 100 mmol / L, pH 7.0 phosphate buffer, and ultrasonically disrupted in an ice bath (power 400W, working 6s, interval 4s, sonicating 99 times). Centrifuge at 8800rpm for 20min to collect the supernatant, which is the crude enzyme solution of the recombina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com