A method for reducing calcium and magnesium ions in electrolytic manganese qualified solution
A technology of electrolytic manganese qualified liquid and calcium and magnesium ions, applied in the field of electrolytic manganese metal hydrometallurgy, to achieve low production and operation costs, obvious removal effect, and avoid low production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
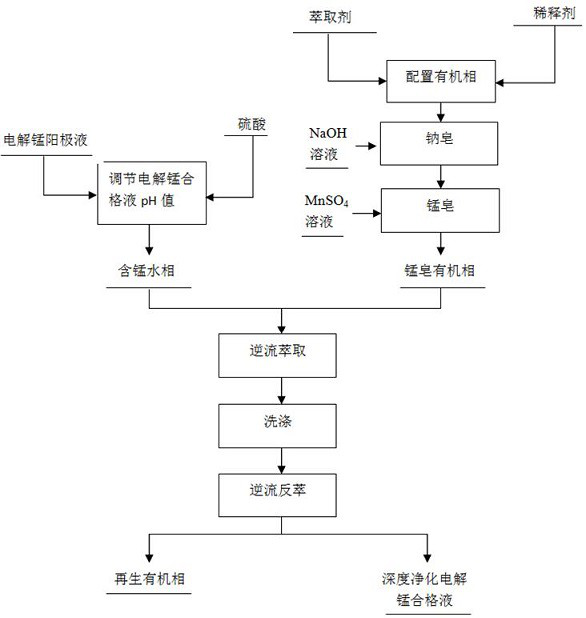
[0024] Such as figure 1 As shown, the method for reducing calcium and magnesium ions in the electrolytic manganese qualified solution, its specific steps include:
[0025] Step 1: Step 1.1, adjust the pH value of the qualified electrolytic manganese solution: adjust the pH value of the qualified electrolytic manganese solution to 2 with 2mol / L sulfuric acid solution, and add the electrolytic manganese anolyte (that is, the electrolytic manganese anolyte, including Mn 2+ 15g / L, Mg 2+ 17g / L, H 2 SO 4 36g / L), the pH-adjusted electrolytic manganese qualified solution includes Mn 2+ 32g / L, Ca 2+ 800mg / L, Mg 2+ The concentration is 38g / L, (NH 4 ) 2 SO 4 130g / L;
[0026] Step 1.2, configure the extraction organic phase: the extraction organic phase is composed of extractant and 260# sulfonated kerosene, the extractant is a combined extractant of P507 and Cyanex272 with a volume ratio of 3:2, and the combined extractant is diluted to this level with 260# sulfonated kerosene ...
Embodiment 2
[0034] Such as figure 1 As shown, the method for reducing calcium and magnesium ions in the electrolytic manganese qualified solution, its specific steps include:
[0035] Step 1: Step 1.1, adjust the pH value of the qualified electrolytic manganese solution: adjust the pH value of the qualified electrolytic manganese solution to 3 with 2mol / L sulfuric acid solution, and add the electrolytic manganese anolyte (that is, the electrolytic manganese anolyte, including Mn 2+ 15g / L, Mg 2+ 17g / L, H 2 SO 4 36g / L), the pH-adjusted electrolytic manganese qualified solution includes Mn 2+ 22g / L, Ca 2+ 400mg / L, Mg 2+ The concentration is 15g / L, (NH 4 ) 2 SO 4 110g / L;
[0036] Step 1.2, configure the extraction organic phase: the extraction organic phase is composed of extractant and 260# sulfonated kerosene, the extractant is a combined extractant of P507 and Cyanex272 with a volume ratio of 3:2, and the combined extractant is diluted to this level with 260# sulfonated kerosene ...
Embodiment 3
[0044] Such as figure 1 As shown, the method for reducing calcium and magnesium ions in the electrolytic manganese qualified solution, its specific steps include:
[0045] Step 1: Step 1.1, adjust the pH value of the qualified electrolytic manganese solution: adjust the pH value of the qualified electrolytic manganese solution to 4 with 2mol / L sulfuric acid solution, and add the electrolytic manganese anolyte (that is, the electrolytic manganese anolyte, including Mn 2+ 15g / L, Mg 2+ 17g / L, H 2 SO 4 36g / L), the pH-adjusted electrolytic manganese qualified solution includes Mn 2+ 20g / L, Ca 2+ 200mg / L, Mg 2+ The concentration is 10g / L, (NH 4 ) 2 SO 4 100g / L;
[0046] Step 1.2, configure the extraction organic phase: the extraction organic phase is composed of extractant and 260# sulfonated kerosene, the extractant is a combined extractant of P507 and Cyanex272 with a volume ratio of 3:2, and the combined extractant is diluted to this level with 260# sulfonated kerosene ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| saponification | aaaaa | aaaaa |
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com