Anti-cataract euphadienol preparation for eyes as well as preparation method and application thereof
A technology of euphanol and ophthalmic preparations, applied in the field of medicine, can solve problems such as cataracts and related ophthalmic diseases that have not been reported in the literature, achieve good prevention and treatment effects, improve permeability, and promote the effect of turbidity absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
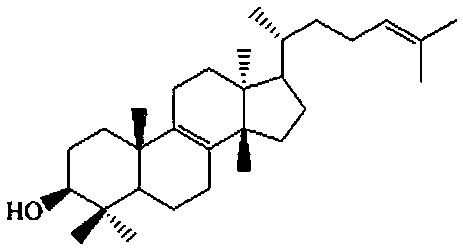
[0052] This embodiment provides an ophthalmic preparation for the prevention and treatment of cataract and related eye diseases, the ophthalmic preparation contains euphanol in a single or combined form.
[0053] Further, the ophthalmic preparation also contains a surfactant and a co-solvent with a pharmaceutical ophthalmic adjuvant, wherein the active ingredient is euphanol.
[0054] Further, the size of the nanoparticles of euphora is 20-100 nanometers.
[0055] Further, the concentration of eugenol in the ophthalmic preparation is 0.01-2.5% (weight / volume).
[0056] Further, said euphorbia can be natural euphorbia, also can be synthetic euphorbia; said natural euphorbia comes from various Euphorbiaceae natural medicines, such as glycerin Sui, Jing Euphorbia, etc.; the synthesis of euphorbia comes from chemical synthesis and biosynthesis.
[0057] Further, the surfactant is one or a mixture of Tween 80, Tween 20, polyethylene glycol ester, polyethylene glycol, lecithin and...
Embodiment 2
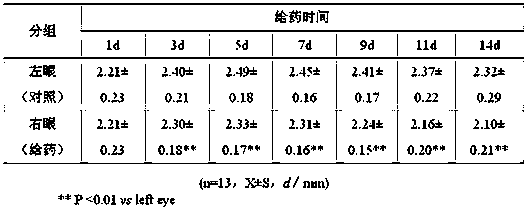
[0075] This embodiment is a preferred scheme based on Example 1. This embodiment provides a kind of eugenol nano-eye drops, and adopts thin film dispersion method to prepare eugenol nano-eye drops liposomes. Orthogonal method takes the encapsulation efficiency measurement results as the optimization index, and prepares the prescription as follows: 200 mg euphanol, 3000 mg lecithin, appropriate amount of absolute ethanol, phosphate buffered saline (PBS) (pH 7.0 ) 100 ml; dissolve the prescribed amount of euphanol and lecithin in absolute ethanol, rotate in vacuum at 40°C to form a thin film, and evaporate until there is no ethanol smell, add 100 ml of PBS (pH value 7.0) , hydration to obtain eugenol liposome suspension. The prepared euphora liposome particles were regular in shape, round or oval, 76.4% of the particles were distributed in the range of 110-200 nm in size, the average particle size was 145.2 nm, and the encapsulation efficiency was 90.02%. The prepared liposome ...
Embodiment 3
[0077]This embodiment is a preferred scheme based on Example 1. This embodiment provides a kind of euphadienol nano eye drops, which adopts thin film dispersion method to prepare euphadienol nano eye drops liposomes. Orthogonal method with encapsulation efficiency measurement results as the optimization index, the preparation of the prescription is as follows: eugenol 1000 mg, lecithin 15000 mg, appropriate amount of absolute ethanol, phosphate buffered saline (PBS) (pH 7.0 ) 500 ml; Dissolve the prescribed amount of euphorbia, lecithin, in absolute ethanol, rotate in vacuum at 40°C to form a thin film, and rotate to evaporate until there is no ethanol smell, add 500 ml of PBS (pH value 7.0) , hydration to obtain eugenol liposome suspension. The prepared euphorbia liposome particles were regular in shape, round or elliptical, 77.8% of them had a particle size distribution between 110 and 200 nm, the average particle size was 145.5 nm, and the encapsulation efficiency was 92.16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com