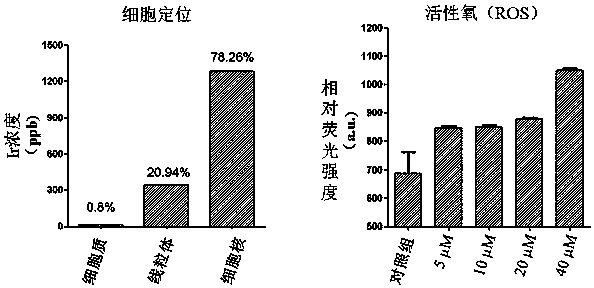
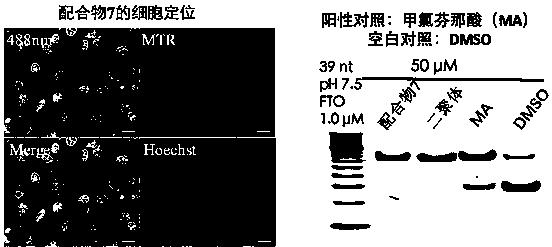
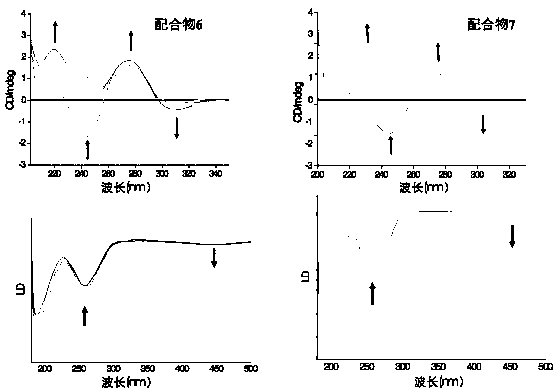
Rhein specific group modified organic compound, aryl metal complex, preparation method and application thereof
A technology of organic compounds and metal complexes, applied in the field of medicine, can solve the problems of FTO inhibitor research reports without metal complexes, achieve high anti-cancer effects, inhibit FTO activity, and increase active oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under argon atmosphere, rhein (0.14g, 0.5mmol), EDCI (1.05g, 5.5mmol), HOBt (0.61g, 4.5mmol) and the bridging ligand 4-(4'-methyl-2, 2'-Bipyridyl)-methylamine (1.0g, 5.0mmol) was dissolved in DMF (N,N-dimethylformamide), and 100 μL of TEA (triethylamine) was added dropwise, and stirred for 5 h under ice bath, The crude product was separated and purified by column chromatography to obtain the organic compound L1 modified by the bridging ligand with a yield of 55%.
[0035] 1 H NMR (400Hz, d 6 -DMSO): 11.93(s,2H), 9.66(t,1H), 8.63(d,1H), 8.52(d,1H), 8.38(s,1H), 8.23(m,2H), 7.85(dd, 2H) 7.77 (d, 1H), 7.42 (t, 2H), 7.28 (d, 1H), 4.63 (d, 2H), 2.42 (s, 3H).
[0036] The preparation method of the above-mentioned bridging ligand 4-(4'-methyl-2,2'-bipyridine)-methylamine is as follows: step (1): SeO 2 (selenium dioxide), 1,4-dioxide (1,4-dioxane), 4,4'-dimethyl-2,2'-bipyridyl (4,4'-dimethyl-2,2'- bipyridyl) mixed in a three-necked flask, reflux heating reaction 0.4-45h, af...
Embodiment 2
[0040] Under argon atmosphere, organic compound L1 (0.05g, 0.1mmol) was mixed with [Ru(η 6 -p-cymene)Cl 2 ] 2 (0.31g, 0.5mmol) was dissolved in methanol, reflux reaction at 65°C for 2h, and NH 4 PF 6 , frozen at -4°C for 1 h, and centrifuged to obtain complex 1 with a yield of 65%.
[0041] 1 H NMR (400Hz, d 6 -DMSO):9.72(s,1H),9.44(d,1H),9.36(d,1H),8.60(s,1H),8.51(s,1H),8.17(s,1H),7.90(s, 1H)7.81(m,1H),7.74(d,1H),7.66(dd,2H),7.41(d,1H),6.19(d,2H),5.94(d,2H),4.75(d,2H) , 3.17(s,2H), 2.59(s,3H), 2.33(m,1H), 2.15(s,3H), 0.95(d,6H). ESI-MS(+): theoretical value: [1-PF 6 - ] + m / z: 736.20, experimental value: m / z 736.25.
Embodiment 3
[0043] Under argon atmosphere, organic compound L1 (0.05g, 0.1mmol) was mixed with [Ru(η 6 -p-cymene)I 2 ] 2 (0.489g, 0.5mmol) was dissolved in methanol, and refluxed at 65°C for 2h. After the reaction, ether was added and centrifuged to obtain complex 2 with a yield of 75%.
[0044] 1 H NMR (400Hz, d 6 -DMSO):11.95(s,2H),9.68(s,1H),9.40(d,1H),9.32(d,1H),8.61(s,1H),8.54(s,1H),8.25(s, 1H)7.88(dd,2H),7.79(d,1H),7.64(dd,2H),7.45(d,1H),6.13(d,2H),6.00(d,2H),4.78(d,2H) ,2.72(m,1H),2.60(s,3H),2.39(s,3H),0.99(d,6H). ESI-MS(+): theoretical value: [2-I - ] + m / z 828.65, experimental value: m / z 828.17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com