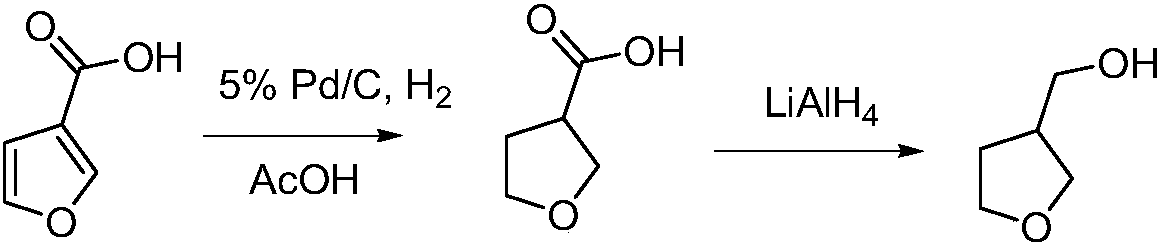
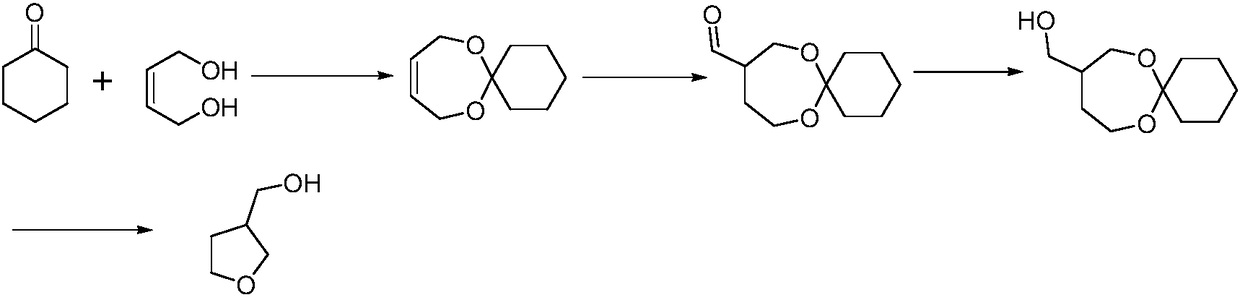
Production technology for 3-hydroxymethyltetrahydrofuran
A technology of hydroxymethyltetrahydrofuran and formyltetrahydrofuran, which is applied in the field of 3-hydroxymethyltetrahydrofuran production technology, can solve the problems of poor atom economy, unfavorable industrial production, cumbersome post-processing, etc., and achieve strong production operability , short steps, no effects of highly toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment includes the following steps:
[0031] The first step, in the reaction kettle, add 8.81kg (100mol) of 1,4-butenediol and 0.615kg (1.2mol) of tris(pentafluorophenyl)borane, react at 135°C for 4.5h, and distill under reduced pressure to obtain 2,5-dihydrofuran 6.71kg (95.7mol), the yield is 95.7%; H NMR (400MHz, CDCl 3 ):5.80(s,2H),4.55(s,4H);
[0032] The second step, in the autoclave, dissolve 6.71kg (95.7mol) of 2,5-dihydrofuran in 9.5L toluene, add RhCl (CO) (DPPB) 8.5g (14.4mmol), NaB (3,5 -(CF 3 ) 2 C 6 h 3 ) 4 12.7g (14.4mmol), after replacing the gas in the kettle with nitrogen for three times, feed H 2 Mix gas with CO (volume ratio 1:1) to a total pressure of 4MPa, heat up to 100°C, react for 2.5h, cool to room temperature, slowly vent, filter, and distill under reduced pressure to obtain 9.03kg (90.15mol ), the yield was 94.2%; H NMR (400MHz, CDCl 3 ):9.60(d,1H),3.92(m,2H),3.87(m,2H),3.05(m,1H),2.16(m,2H);
[0033] The 3rd step, add 3-f...
Embodiment 2
[0035] This embodiment includes the following steps:
[0036] The first step, in the reaction kettle, add 8.81kg (100mol) of 1,4-butenediol and 1.024kg (2mol) of tris(pentafluorophenyl)borane, react at 125°C for 5h, and distill under reduced pressure to obtain 2, 5-dihydrofuran 6.48kg (92.5mol), the yield is 92.5%;
[0037] In the second step, in an autoclave, dissolve 6.48kg (92.5mol) of 2,5-dihydrofuran in 9L of toluene, add 6.5g (11.1mmol) of RhCl(CO)(DPPB), NaB(3,5- (CF 3 ) 2 C 6 h 3 ) 4 9.8g (11.1mmol), after replacing the gas in the kettle with nitrogen for three times, feed H 2 Mix gas with CO (volume ratio 1:1) to a total pressure of 4MPa, heat up to 90°C, react for 4h, cool to room temperature, slowly vent, filter, and distill under reduced pressure to obtain 8.87kg (88.6mol) of 3-formyltetrahydrofuran , the yield is 95.8%;
[0038] The third step, add 3-formyl tetrahydrofuran 8.87kg (88.6mol) in the autoclave, 5%Pd / C 0.44kg, and 100L ethanol, after three time...
Embodiment 3
[0040] This embodiment includes the following steps:
[0041]The first step, in the reaction kettle, add 8.81kg (100mol) of 1,4-butenediol and 0.512kg (1mol) of tris(pentafluorophenyl)borane, react at 125°C for 6h, and distill under reduced pressure to obtain 2, 5-dihydrofuran 6.81kg (97.1mol), the yield is 97.1%;
[0042] In the second step, in an autoclave, dissolve 6.81kg (97.1mol) of 2,5-dihydrofuran in 10L of toluene, add 5.7g (9.71mmol) of RhCl(CO)(DPPB), NaB(3,5- (CF 3 ) 2 C 6 h 3 ) 4 8.5g (9.71mmol), after replacing the gas in the kettle with nitrogen for three times, feed H 2 Mix gas with CO (volume ratio 1:1) to a total pressure of 4MPa, heat up to 95°C, react for 3h, cool to room temperature, slowly vent, filter, and distill under reduced pressure to obtain 9.39kg (93.8mol) of 3-formyl tetrahydrofuran , the yield is 96.6%;
[0043] The third step, add 3-formyl tetrahydrofuran 9.39kg (93.8mol) in the autoclave, 5%Pd / C 0.47kg, and 110L ethanol, after the gas i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com