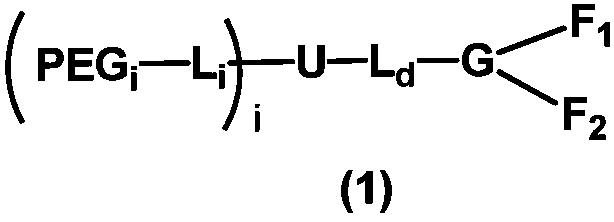Branched polyethylene glycol iso-bifunctional derivative, as well as preparation method and two-component biological related substance conjugate thereof
A bifunctional, polyethylene glycol technology, applied in the field of two-component bio-related substance conjugates and branched polyethylene glycol heterobifunctional derivatives, can solve the problem of prolonging the action time of drugs and unable to target positioning. function, increase steric hindrance, etc., to reduce the degree of inactivation or hydrolysis by enzymes, prolong the action time, and increase the effect of steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0271] For related preparation methods, reference may be made to the preparation methods and reaction conditions in the prior art. Including but not limited to the types of chemical reactions involved in documents CN104530417A, CN104877127A, WO / 2016 / 206540A, CN201610252378X and the cited documents.
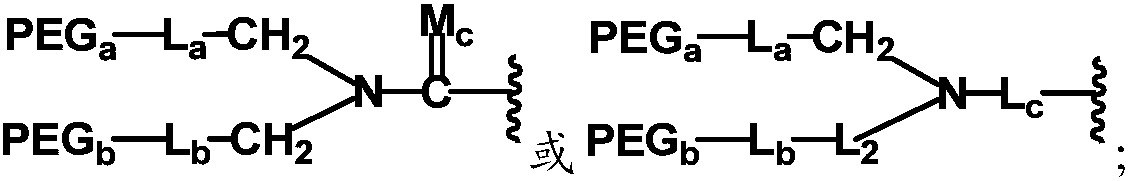
[0272] For those who do not have a suitable pre-modification form for the target functional group when coupling the nitrogen branching center and the polyethylene glycol chain, it is also possible to conduct a pair of terminal heterofunctional groups based on the prepared branched polyethylene glycol heterobifunctional derivatives. Chemical modification is achieved. The suitable pre-modified form refers to the one that can be converted into the target functional group through micro-modification, and can keep the structure unchanged during the preparation process, especially when the azo branching center and the polyethylene glycol chain are coupled, without causing side reactions ...
Embodiment 1
[1062] Embodiment 1 Lysine branched polyethylene glycol derivative (α-N)
[1063]
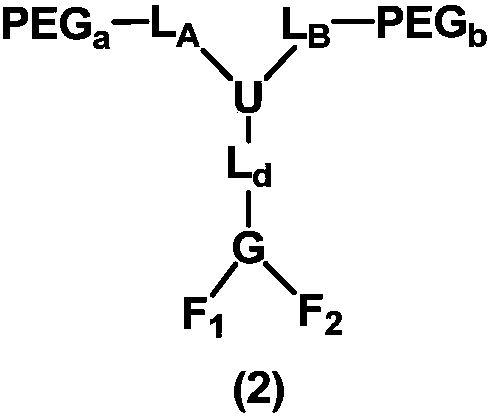
[1064] Among them, the corresponding general formula (2) PEG a 、PEG b Both are mPEG structure, L A , L B Both are ethylene groups, U is a nitrogen atom, L d Does not exist, G is a tertiary carbon, F 1 , F 2 One of them is COOH, the other is (CH 2 ) 4 NH 2 , with F 1 for COOH, F 2 for (CH 2 ) 4 NH 2 For example, R 01 for COOH, Z 1 does not exist, R 02 for NH 2 ,Z 2 For 1,4-butylene. Both PEGs have a molecular weight of 20 kDa, for a total molecular weight of approximately 40 kDa.
[1065] Step a: Lys(Cbz)-OH hydrochloride was washed with a small amount of water, and dried to obtain mono-protected lysine S1-1 (Lys(Cbz)-OH) from which the hydrochloride was removed. 1 H NMR (CDCl 3 ): 1.24-1.60 (-CH 2 CH 2 CH 2 CH2 NH-Cbz,2H),5.10(Cbz,-CH 2 -, 2H), 7.2 to 7.4 (Cbz, Ph-, 5H).
[1066] Step b: Alkylation reaction of 30 mmol monoprotected lysine small molecule S1-1 with me...
Embodiment 2
[1070] Embodiment 2 glycine branched polyethylene glycol derivatives
[1071]
[1072] Among them, the corresponding general formula (2) PEG a 、PEG b Both are mPEG structure, L A , L B Both are ethylene groups, U is a nitrogen atom, L d for CH 2 CONH, G is tertiary carbon, F 1 , F 2 One of them is COOH, the other is (CH 2 ) 4 NH 2 , with F 1 for COOH, F 2 for (CH 2 ) 4 NH 2 For example, R 01 for COOH, Z 1 does not exist, R 02 for NH 2 ,Z 2 For 1,4-butylene. Both PEGs have a molecular weight of 10 kDa, for a total molecular weight of about 20 kDa.
[1073] Step a: Lys(Cbz)-OMe hydrochloride was washed with a small amount of water, and dried to obtain mono-protected lysine S2-1 (Lys(Cbz)-OMe) from which the hydrochloride was removed. 1 H NMR (CDCl 3 ): 1.24-1.60 (-CH 2 CH 2 CH 2 CH2 NH-Cbz,2H),3.72(-COOCH 3 ,3H),5.10(Cbz,-CH 2 -, 2H), 7.2 to 7.4 (Cbz, Ph-, 5H).
[1074] Step b: Alkylation reaction of 30mmol glycine and methoxypolyethylene glycol me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com