Anti-tumor nano-drug
A technology of nano-drugs and nano-drug carriers, which is applied in the direction of anti-tumor drugs, drug combinations, drug delivery, etc. It can solve the problems of proportional entry into cells, difficult to achieve, and poor curative effect, so as to improve curative effect, avoid large toxic and side effects, and not easy to remove Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of lipoic acid polymers.
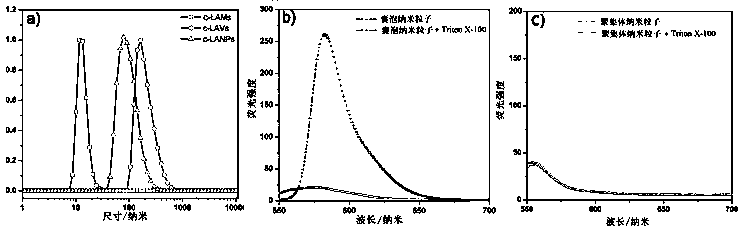
[0060] Lipoic acid polymers can exist in the form of lipoic acid micelles, lipoic acid vesicles and lipoic acid aggregates, such as figure 1 Shown.
[0061] 1. Preparation of lipoic acid micelles (Cross-linked lipoic acid micelles, c-LAMs):
[0062] Add 100 mg of lipoic acid (LA) to 50 ml of deionized water, add 1 M NaOH aqueous solution dropwise with stirring until the lipoic acid is completely dissolved, then drop 1 M HCl solution until the solution is neutral, and finally freeze the solution. A pale yellow sodium lipoic acid powder was obtained. Weigh 41.2 mg (0.2 mol) of sodium lipoic acid, dissolve it in 1 ml of deionized water, and prepare nanoparticles with a size of about 15 nm after ultrasound. The nanoparticles obtained above were subjected to 365 nm ultraviolet light to initiate self-crosslinking of lipoic acid disulfide bonds, reacted for 2.5 h, and after dialysis for 48 h, cross-linked lipoic acid micelles (Cross-linked lip...
Embodiment 2
[0071] Study on the anti-tumor mechanism of lipoic acid polymer.
[0072] Select cross-linked lipoic acid micelles (c-LAMs) as an example to study the anti-tumor activity mechanism of lipoic acid polymers.
[0073] 1. Cytotoxicity assessment:
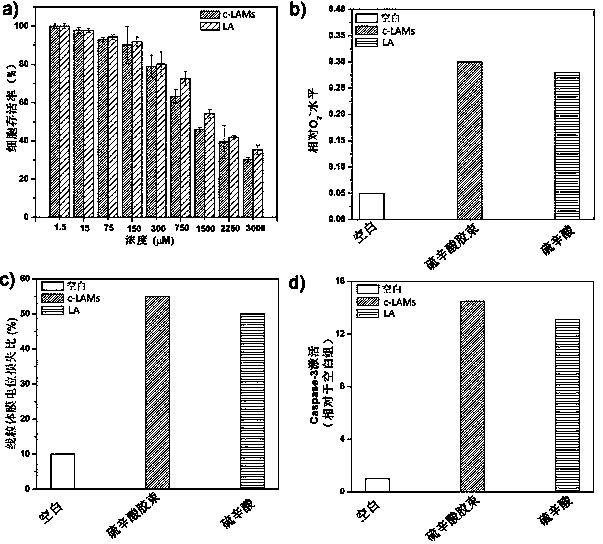
[0074] Select human colon cancer cells (SW480) in the active logarithmic growth phase, inoculate them in 96-well plates, culture for 24 hours, then add different concentrations of LA and c-LAMs to them, set different concentration gradients, and set each concentration setting 5 parallel samples and a control group. After culturing for 48 hours, remove the medium, add 100 μl of medium containing 10% (v / v) MTT and continue incubating for 2 hours, then aspirate the old medium, and then add 150 μl of DMSO to each well, and Oscillate on a shaker for 2 minutes, and finally measure the absorbance at 490 nm with a microplate reader to calculate the cell viability. The result is as follows image 3 shown in a. The experimental results found that c-L...
Embodiment 3
[0084] Evaluation of lipoic acid polymer and LA anti-tumor activity.
[0085] Choose lipoic acid micelles (c-LAMs) as an example to evaluate the anti-tumor activity of lipoic acid polymers and LA.
[0086] Human hepatocellular carcinoma cells (HepG2) in the active logarithmic growth phase were selected and seeded in 96-well plates. After 24 hours of culture, c-LAMs and LA were added. Different concentration gradients are set for the two materials, 5 parallel samples are set for each concentration, and a control group is set. After 72 hours of incubation, aspirate the old medium, add 100 μl of medium containing 10% (v / v) MTT to each well, incubate for 2 hours, aspirate the old medium, add 150 μl of DMSO to each well, and shake After oscillating on the device for 2 minutes, measure the absorbance at 490 nm with a microplate reader to calculate the cell viability. The experimental results are as follows Figure 4 As shown, c-LAMs has better anti-tumor activity than LA. The reason may...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com