Method for removing CO2 from biohydrogen alkane gas by ionic liquid supported on graphene
A technology of ionic liquid and biohydroane, applied in separation methods, chemical instruments and methods, gas fuels, etc., can solve the problems of high velocity and viscosity, and achieve the effect of increasing the absorption reaction rate and shortening the absorption equilibrium time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
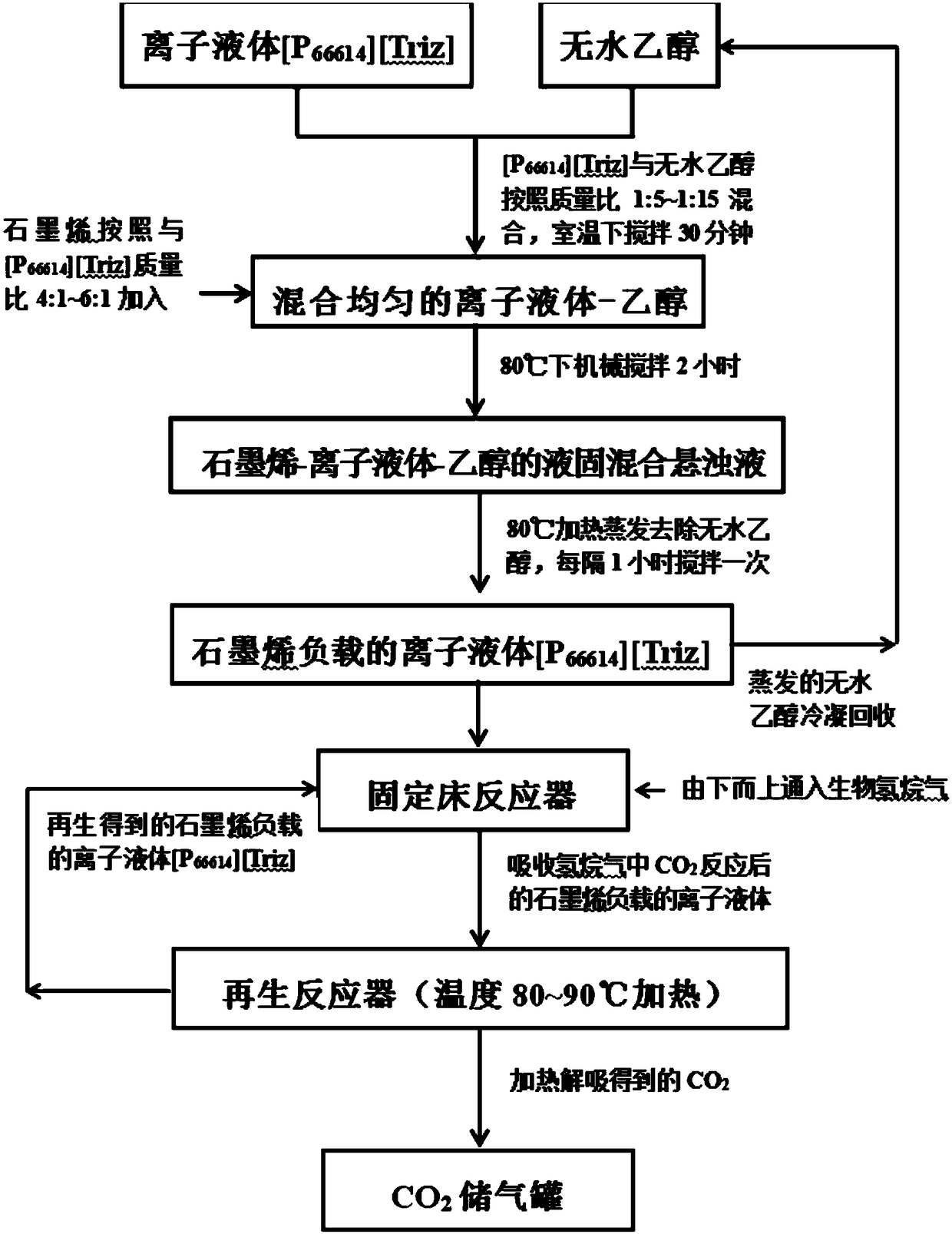
[0022] will absorb CO 2 Capacity is 0.95mol CO 2 / mol of ionic liquid [P 66614 ][Triz] was mixed with absolute ethanol at a mass ratio of 1:5, and mechanically stirred at room temperature for 30 minutes to obtain a uniformly mixed ionic liquid-ethanol solution. Then graphene (specific surface area is 1000m 2 / g) in accordance with the ionic liquid [P 66614 ][Triz] with a mass ratio of 4:1 was added to the ionic liquid-ethanol solution, and mechanically stirred at 80°C for 2 hours. Reflux the evaporated absolute ethanol with a condenser to obtain a mixed liquid-solid suspension of graphene-ionic liquid-ethanol. The liquid-solid mixed suspension was heated and evaporated at 80°C to remove absolute ethanol, and stirred every 1 hour during the evaporation process to obtain the ionic liquid [P 66614 ][Triz]. The evaporated anhydrous ethanol is recycled after being condensed and recovered. Ionic liquids loaded on graphene [P 66614 ][Triz] into a fixed bed reactor. At the bo...
Embodiment 2
[0024] will absorb CO 2 Capacity is 0.95mol CO 2 / mol of ionic liquid [P 66614 ][Triz] was mixed with absolute ethanol at a mass ratio of 1:10, and mechanically stirred at room temperature for 30 minutes to obtain a uniformly mixed ionic liquid-ethanol solution. Then graphene (specific surface area is 800m 2 / g) in accordance with the ionic liquid [P 66614 ][Triz] with a mass ratio of 5:1 was added to the ionic liquid-ethanol solution, and mechanically stirred at 80°C for 2 hours. Reflux the evaporated absolute ethanol with a condenser to obtain a mixed liquid-solid suspension of graphene-ionic liquid-ethanol. The liquid-solid mixed suspension was heated and evaporated at 80°C to remove absolute ethanol, and stirred every 1 hour during the evaporation process to obtain the ionic liquid [P 66614 ][Triz]. The evaporated anhydrous ethanol is recycled after being condensed and recovered. Ionic liquids loaded on graphene [P 66614 ][Triz] into a fixed bed reactor. At the bo...
Embodiment 3
[0026] will absorb CO 2 Capacity is 0.95mol CO 2 / mol of ionic liquid [P 66614 ][Triz] was mixed with absolute ethanol at a mass ratio of 1:15, and mechanically stirred at room temperature for 30 minutes to obtain a uniformly mixed ionic liquid-ethanol solution. Then graphene (specific surface area is 600m 2 / g) in accordance with the ionic liquid [P 66614 ][Triz] with a mass ratio of 6:1 was added to the ionic liquid-ethanol solution, and mechanically stirred at 80°C for 2 hours. Reflux the evaporated absolute ethanol with a condenser to obtain a mixed liquid-solid suspension of graphene-ionic liquid-ethanol. The liquid-solid mixed suspension was heated and evaporated at 80°C to remove absolute ethanol, and stirred every 1 hour during the evaporation process to obtain the ionic liquid [P 66614 ][Triz]. The evaporated anhydrous ethanol is recycled after being condensed and recovered. Ionic liquids loaded on graphene [P 66614 ][Triz] into a fixed bed reactor. At the bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com