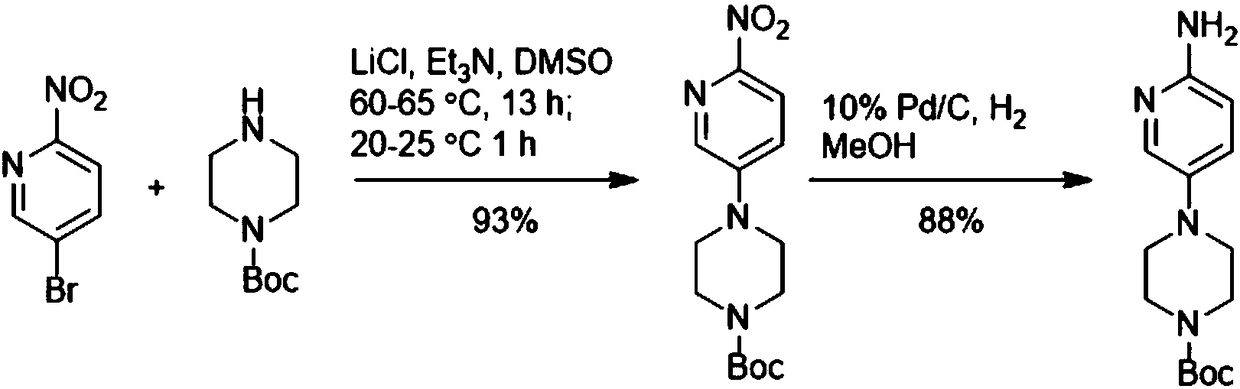
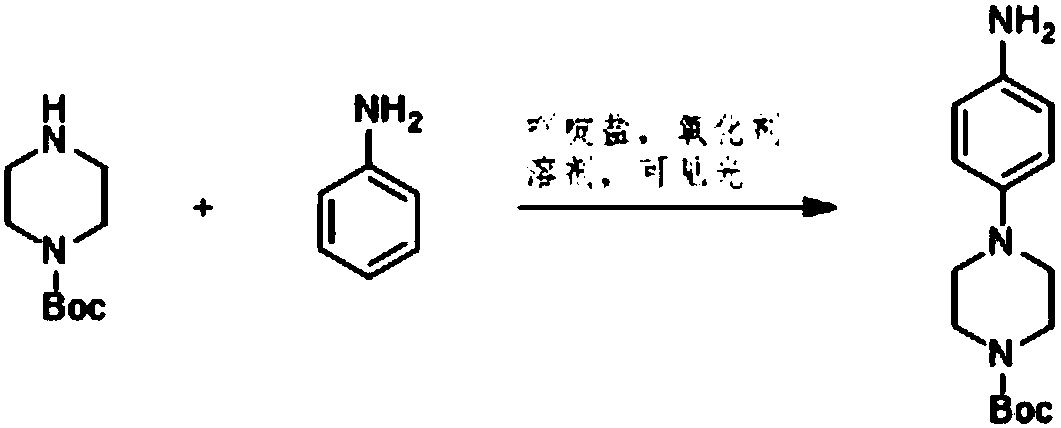
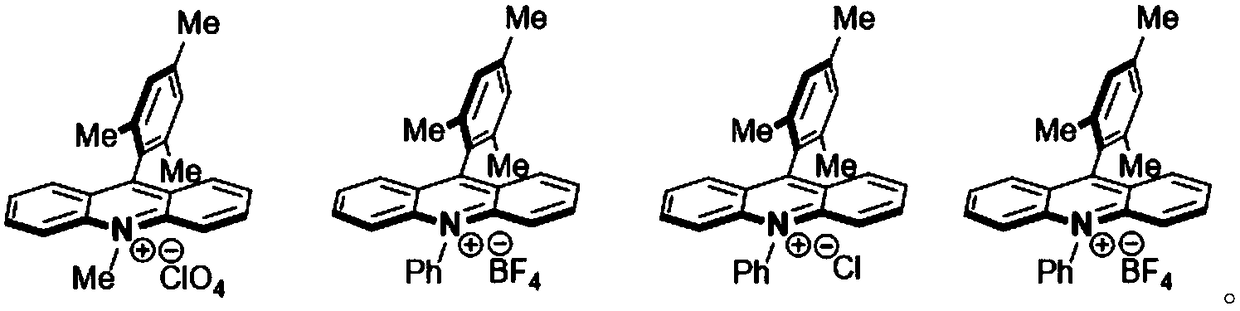
Method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester
A technology of tert-butyl carboxylate and aminopyridine, which is applied in the field of preparation of 4-piperazine-1-tert-butyl carboxylate, can solve the problems of low yield of target product, many by-products, less than comprehensive yield and the like , to avoid the use of heavy metals and hydrogen environment, reduce the generation of by-products, and shorten the synthesis path.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] The invention provides a preparation method of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The preparation method of 4-(6-aminopyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester includes the following steps:
[0012] Add 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, and acridine salt photocatalyst into the solvent, and carry out light reaction in the presence of oxidant to generate 4-(6-aminopyridin-3-yl) Tert-butyl piperazine-1-carboxylate.
[0013] Specifically, in the light reaction, the 2-aminopyridine and piperazine-1-carboxylic acid tert-butyl ester are catalyzed by light and an acridine salt photocatalyst and in the presence of an oxidizing agent to form a product compound C. The chemical reaction formula of the light reaction is as follows:
[0014]
[0015] Wherein, the acridine salt promotes the condensation reaction of the 2-aminopyridine and the tert-butyl piperazine-1-carboxylate under light conditions. In one embodime...
Embodiment 1
[0026] This embodiment provides a method for preparing tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The synthesis method of 4-(6-aminopyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester:
[0027] Add 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, acridine salt visible light catalyst, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide to anhydrous dichloroethane, Then, the reaction environment was replaced with oxygen three times, and the blue LED was irradiated, and the reaction time was 10 hours. After the completion of the reaction, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 95%.
[0028] Wherein, the 2-aminopyridine, tert-butyl piperazine-1-carboxylate, acridine salt, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide and anhydrous dichloroethane are in accordance with Add in the following proportions: for every 2mL of anhydrous dichloroethane, add 0.2mmol...
Embodiment 2
[0033] This embodiment provides a method for preparing tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The preparation method of the m-aminophenylacetylene is as follows:
[0034] Add 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, acridine salt visible light catalyst, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide to anhydrous dichloroethane Then, the reaction environment is replaced with oxygen three times, and the blue LED is irradiated. The reaction time is 10h. After the reaction, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 92%.
[0035] Wherein, the 2-aminopyridine, tert-butyl piperazine-1-carboxylate, acridine salt, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide and anhydrous dichloroethyl The alkane is added in the following proportions: for every 2mL of anhydrous dichloroethane, add 0.2mmol, 1.0eq of 2-aminopyridine, 0.2mmol, 1.0eq of tert-butyl pipe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com