Substituted heteroaryl compound and composition and application thereof
A technology for compounds and heterocyclic groups, which can be used in drug combinations, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as high infection sensitivity and tumor surveillance defects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
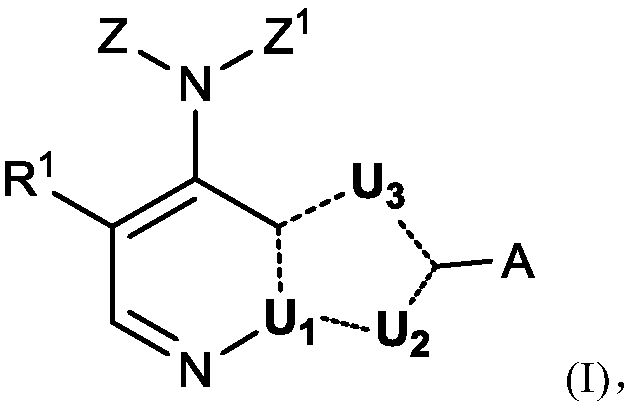
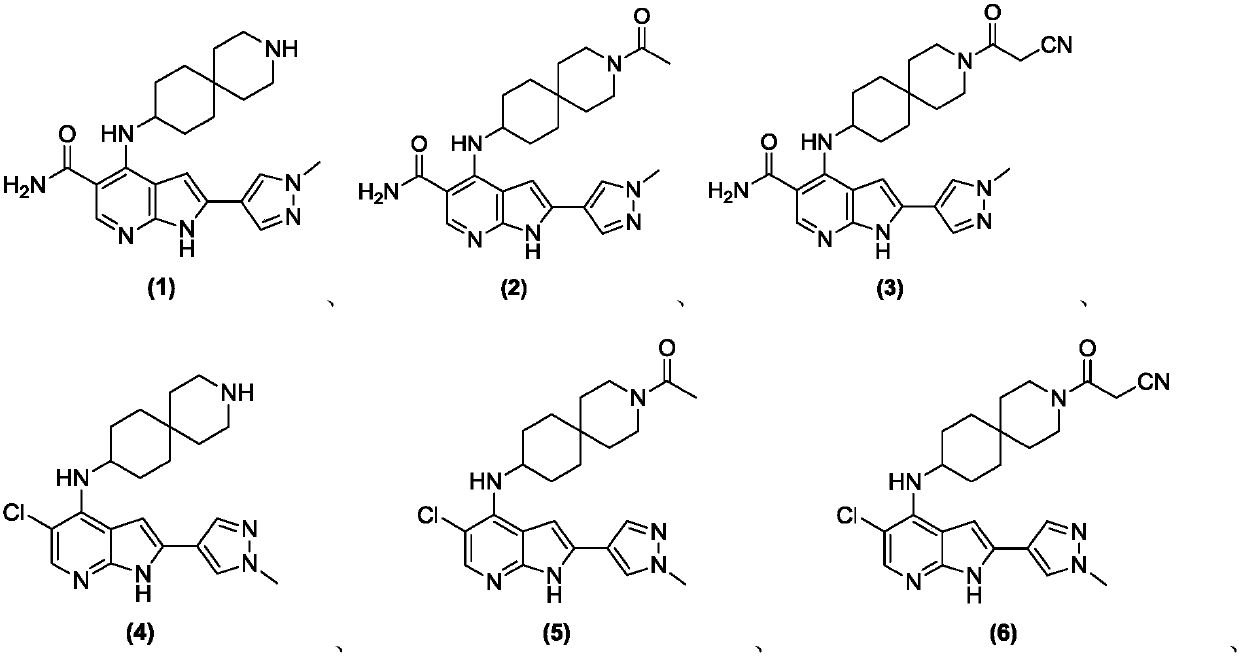
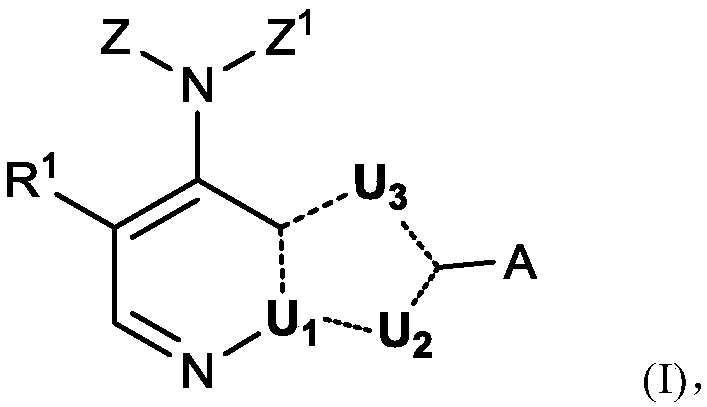
[0480] Example 1 4-(3-Azaspiro[5.5]undecan-9-ylamino)-2-(1-methyl-1H-pyrazol-4-yl)-1H- Pyrrolo[2,3-b]pyridine-5-carboxamide
[0481]
[0482] Step 1) 4-Chloro-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine
[0483] To a suspension of sodium hydride (60% [w / w] mass fraction, suspended in mineral oil, 2.68 g, 67.0 mmol) in tetrahydrofuran (60 mL) at 0 °C, 4-chloro-1H-pyrrole was added dropwise A solution of iso[2,3-b]pyridine (8.24 g, 54.0 mmol) in tetrahydrofuran (80 mL). After the resulting reaction mixture was stirred at room temperature for 30 minutes, tris(isopropyl)chlorosilane (12.73 g, 66.0 mmol) was added to the above system. The reaction system was stirred at room temperature for 5 hours, and then concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (100% PE) to give the title compound as a colorless oil (16.68 g, yield 100%).
[0484] MS(ESI,pos.ion)m / z:309.0[M+H] + ;
[0485] 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0515] Example 2 4-((3-Acetyl-3-azaspiro[5.5]undecan-9-yl)amino)-2-(1-methyl-1H-pyridine oxazol-4-yl)-1H-pyrrolo[2,3-b]pyridine-5-carboxamide
[0516]
[0517] 4-(3-Azaspiro[5.5]undecan-9-ylamino)-2-(1-methyl-1H-pyrazol-4-yl)-1H-pyrrolo[2,3-b ] Pyridine-5-carboxamide (110.1 mg, 0.27 mmol) and triethylamine (76.4 mg, 0.76 mmol) were suspended in dichloromethane (8 mL), and acetic anhydride (34.6 mg, 0.34 mmol) was added to the suspension. ) in dichloromethane (2 mL). The reaction system was stirred at room temperature for 20 minutes, then water (50 mL) was added to quench the reaction and extracted with dichloromethane (150 mL x 6). The combined organic phases were washed with saturated brine (150 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (MeOH / DCM (v / v)=1 / 5) to obtain the title compound as an off-white solid (64.0 mg, yield 52.7%).
[0518] MS(ESI,p...
Embodiment 3
[0520] Example 3 4-((3-(2-cyanoacetyl)-3-azaspiro[5.5]undecan-9-yl)amino)-2-(1-methyl) yl-1H-pyrazol-4-yl)-1H-pyrrolo[2,3-b]pyridine-5-carboxamide
[0521]
[0522] To 4-(3-azaspiro[5.5]undecan-9-ylamino)-2-(1-methyl-1H-pyrazol-4-yl)-1H-pyrrolo[2,3-b ] Pyridine-5-carboxamide (0.55 g, 1.30 mmol) and 2-cyanoacetic acid (171.0 mg, 2.01 mmol) in dichloromethane (40 mL) and DMF (10 mL) was added HATU (758.4 mg) , 2.00 mmol) and triethylamine (0.35 g, 3.50 mmol). The reaction system was stirred at room temperature for 10 minutes, then water (50 mL) was added to quench the reaction, and it was extracted with dichloromethane (150 mL x 3). The combined organic phases were washed with saturated brine (150 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (MeOH / DCM (v / v)=1 / 5) to obtain the title compound as an off-white solid (54.4 mg, yield 8.5%).
[0523] MS(ESI,pos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com