Method for leaching cobalt by synergetic reduction of cobalt ores
A cobalt ore and leaching technology is applied in the field of cobalt ore co-reduction leaching, which can solve the problems of low leaching rate, high cost, and environmental hazards caused by sulfur dioxide escape, and achieve the effect of improving leaching rate and realizing recycling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
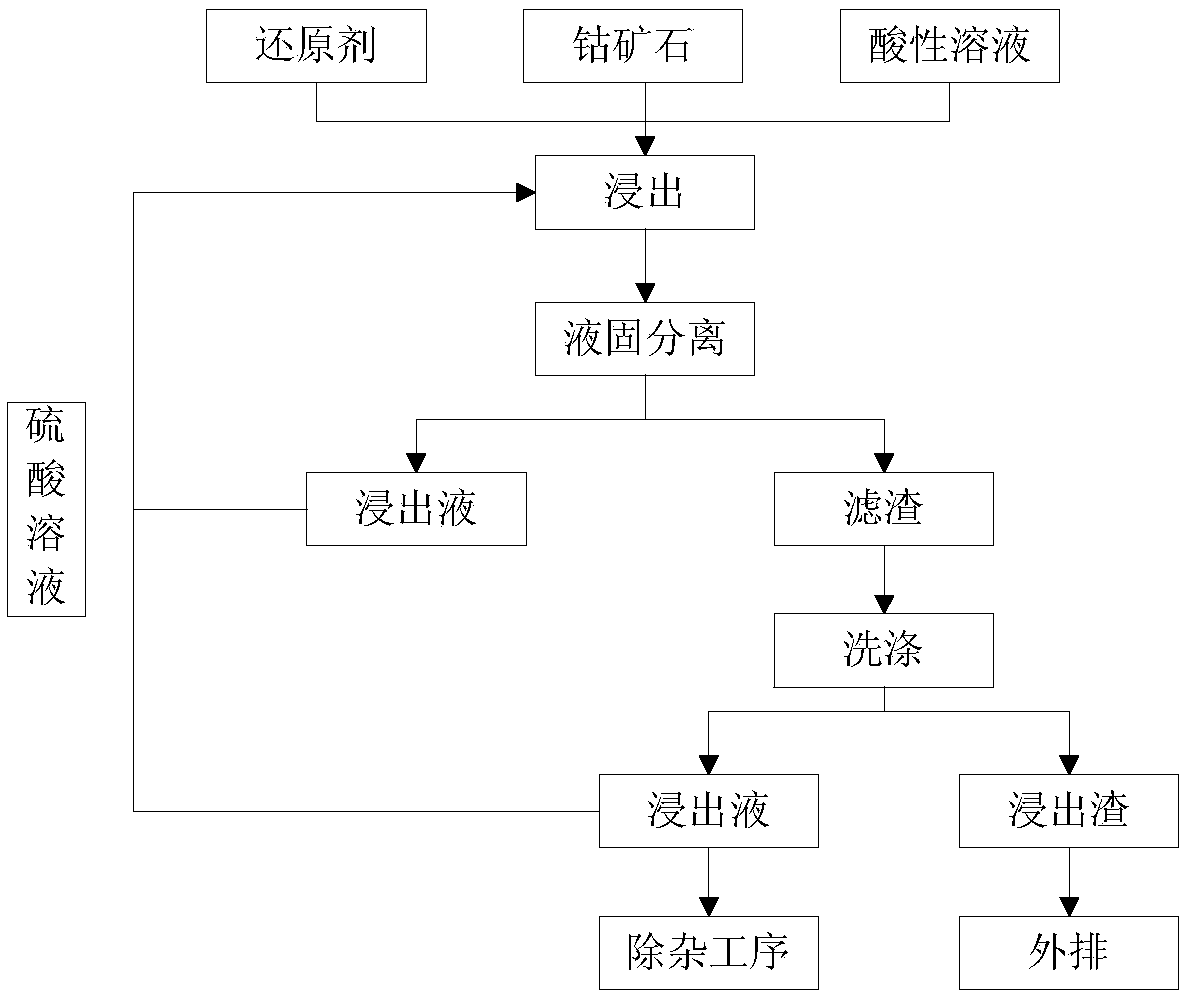
[0040] The weight percentage of cobalt in the Congo (golden) cobalt ore of this embodiment is 0.69%, and the weight percentage of copper is 0.93%. 90m of sulfuric acid solution containing ferrous ion concentration of 1g / L 3 / h, cobalt ore 30t / h mixed (sulfuric acid solution and cobalt ore are continuously input at the above speed), stirred and leached for 8h at room temperature, and SO2 was introduced during the leaching process 2 Gas reaction, the pH value at the end point of the reaction is 1.74, and the end point potential is 290mv. The end point reaction solution is subjected to liquid-solid separation, and the obtained filter residue is washed twice with sulfuric acid solution. According to the above steps, cyclic leaching is performed 3 times to obtain a leaching solution containing cobalt ions.
[0041] The above-mentioned leachate containing cobalt ions can increase the cobalt ion concentration to 4.5-6g / L after cyclic leaching and use, and the crude cobalt hydroxide ...
Embodiment 2
[0044] The weight percentage of cobalt in the Congo (golden) cobalt ore of this embodiment is 0.98%, and the weight percentage of copper is 2.93%. 130m of sulfuric acid solution containing ferrous ion concentration 2.5g / L 3 / h, cobalt ore 33t / h mixed (sulfuric acid solution and cobalt ore are continuously input at the above speed), stirred and leached for 6h at room temperature, and SO4 was introduced during the leaching process 2 gas, the pH value at the end point of the leaching reaction is 1.57, and the end point potential is 307mv. The end point reaction solution is subjected to liquid-solid separation, and the obtained filter residue is washed twice with sulfuric acid solution. According to the above steps, the leaching cycle is performed twice to obtain a leach solution containing cobalt ions.
[0045] The above-mentioned leachate containing cobalt ions can increase the cobalt ion concentration to 4.5-6g / L after cyclic leaching and use, and the crude cobalt hydroxide pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com