Layered perovskite-type oxide, preparation method and use thereof in the oxygen evolution reaction electrocatalyst
A perovskite oxide, layered technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of high price, shortage of resources and poor stability and other problems, to achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 1. Preparation of catalyst slurry: A 3 B 2 o 7-δ The powder is mixed with a certain amount of conductive carbon black (the mass fraction of conductive carbon black is 10%-90%, which plays the role of conductivity and carrier, and has almost no catalytic effect) dispersed in ethanol or other organic solvents, and then add an appropriate amount of Nafion to it (mass fraction 5%) solution, (catalyst concentration is 1-10 mg / mL, Nafion concentration in the solution is 0.2-2 mg / mL), and then ultrasonically oscillated to mix evenly to obtain a catalyst slurry.
[0042] 2. Electrode preparation: pipette a certain amount of the above-prepared catalyst slurry onto the rotating disk electrode (RDE) with a micro-syringe, so that the loading amount on the RDE is 0.1-1 mg / cm 2 , then air dry naturally.
[0043] 3. Measurement of electrode activity: The prepared electrode was installed on a rotating disc device (Pine Company) for electrochemical testing.
[0044] The testing proc...
Embodiment 1
[0049] Example 1 Sr 3 (Co 0.8 Fe 0.2 ) 2 o 7-δ Catalyst preparation and evaluation of catalytic activity for oxygen evolution
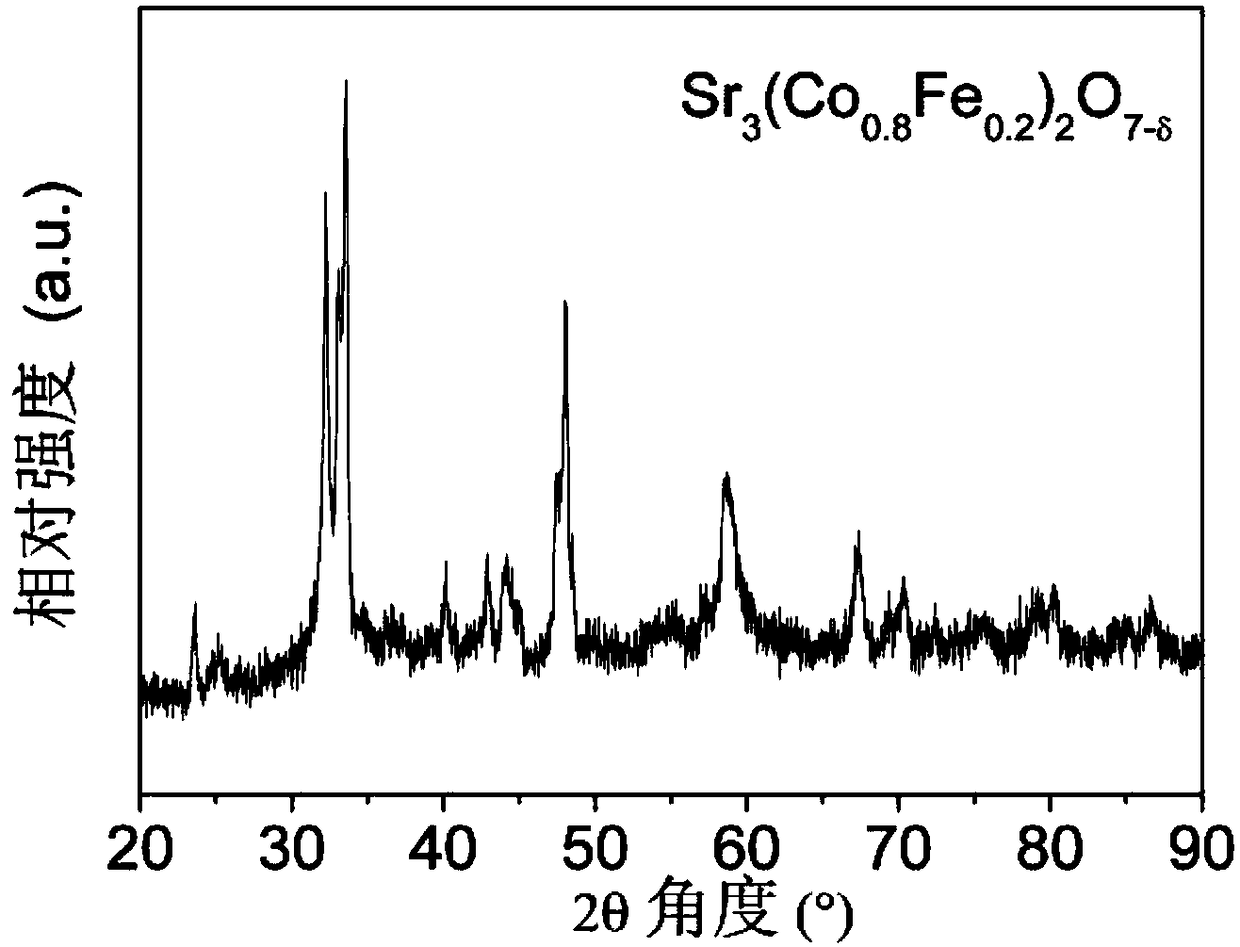
[0050] Sr 3 (Co 0.8 Fe 0.2 ) 2 o 7-δ Catalyst powder is synthesized by solid state reaction method. Weigh the stoichiometric ratio of SrCO 3 (analytical pure), Co 3 o 4 (analytical pure) and Fe 3 o 4 (analytical pure), dissolved in ethanol (analytical pure) medium, stirred and ball milled for 2 h until the mixture was uniform, then further dried to evaporate the medium to obtain a solid precursor, and finally placed the precursor in a muffle furnace at 900 ° C for 10 h, that is, the required Sr 3 (Co 0.8 Fe 0.2 ) 2 o 7-δ catalyst powder. figure 1 The X-ray diffraction (XRD) profile shown indicates that Sr 3 (Co 0.8 Fe 0.2 ) 2 o 7-δ A Ruddlesden-Popper layered structure is formed.
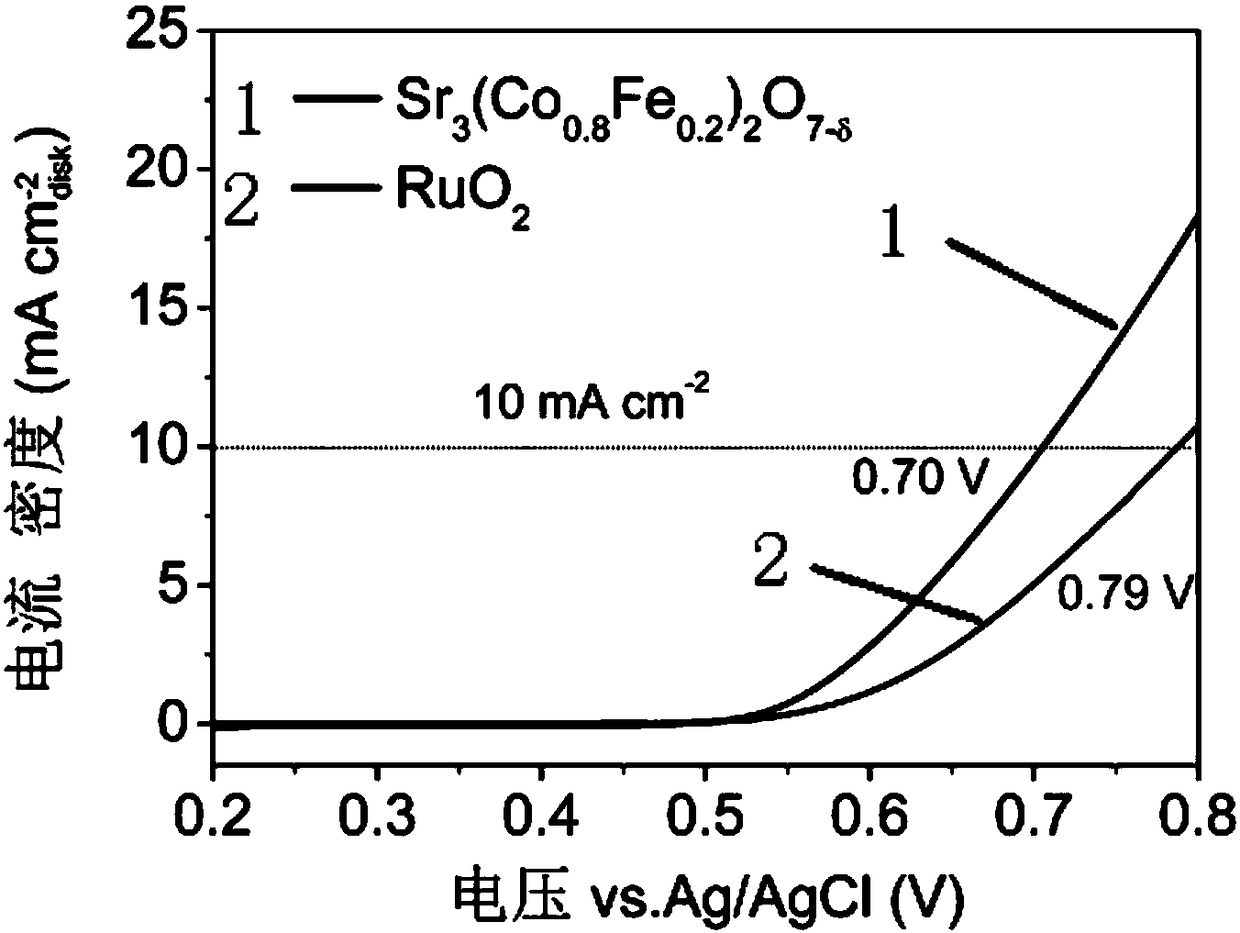
[0051] Oxygen evolution catalytic performance evaluation of catalysts. Weigh 10 mg Sr 3 (Co 0.8 Fe 0.2 ) 2 o 7-δ The catalyst and 10 mg o...
Embodiment 2
[0053] Example 2 Sr 3 (Co 0.9 Nb 0.1 ) 2 o 7-δ Catalyst preparation and evaluation of catalytic activity for oxygen evolution
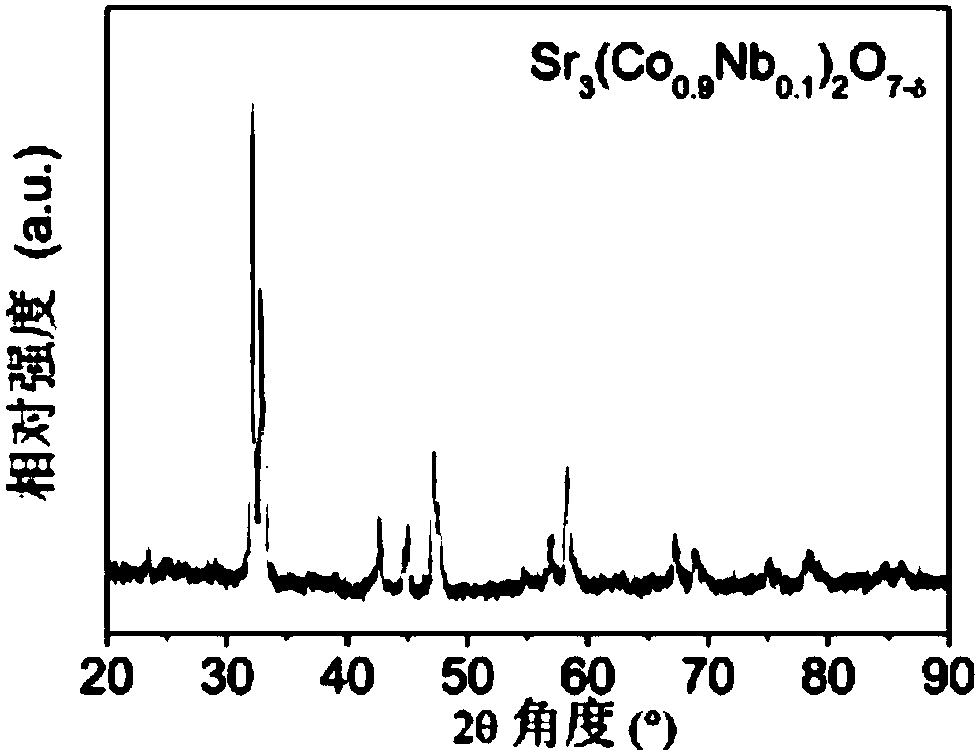
[0054] Sr 3 (Co 0.9 Nb 0.1 ) 2 o 7-δ Catalyst powder is synthesized by solid state reaction method. Weigh the stoichiometric ratio of SrCO 3 (analytical pure), Co 3 o 4 (analytically pure) and Nb 2 o 5 (analytical pure), dissolved in ethanol (analytical pure) medium, stirred and ball-milled for 2 h until the mixture was uniform, then further dried to evaporate the medium to obtain a solid precursor, and finally placed the precursor in a muffle furnace and roasted at 1000 °C for 10 h, that is, the required Sr 3 (Co 0.9 Nb 0.1 ) 2 o 7-δ catalyst powder. image 3 The X-ray diffraction (XRD) profile shown indicates that Sr 3 (Co 0.9 Nb 0.1 ) 2 o 7-δ A Ruddlesden-Popper layered structure is formed.
[0055] Oxygen evolution catalytic activity evaluation of catalysts. The electrode preparation and electrode testing process are t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com