Pharmaceutical composition including dorzolamide and brimonidine
A technology of brimonidine and composition, applied in the field of pharmaceutical compositions containing dorzolamide and brimonidine, can solve problems such as corneal epithelial diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0106] Although the formulation example and the result of the antiseptic efficacy test are shown below, it is for better understanding of this invention, and does not limit the scope of this invention.
[0107] Preparation example
[0108] Representative formulation examples of the present invention are shown below. In addition, the compounding quantity of each component in the following formulation example is the content in 1 mL of formulations.
preparation example 2
[0111] Formulation example 2 (in multi-dose container)
[0112]
[0113] It should be noted that the desired composition can be obtained by appropriately adjusting the types and amounts of dorzolamide, brimonidine, and additives in the aforementioned formulation examples 1 and 2.
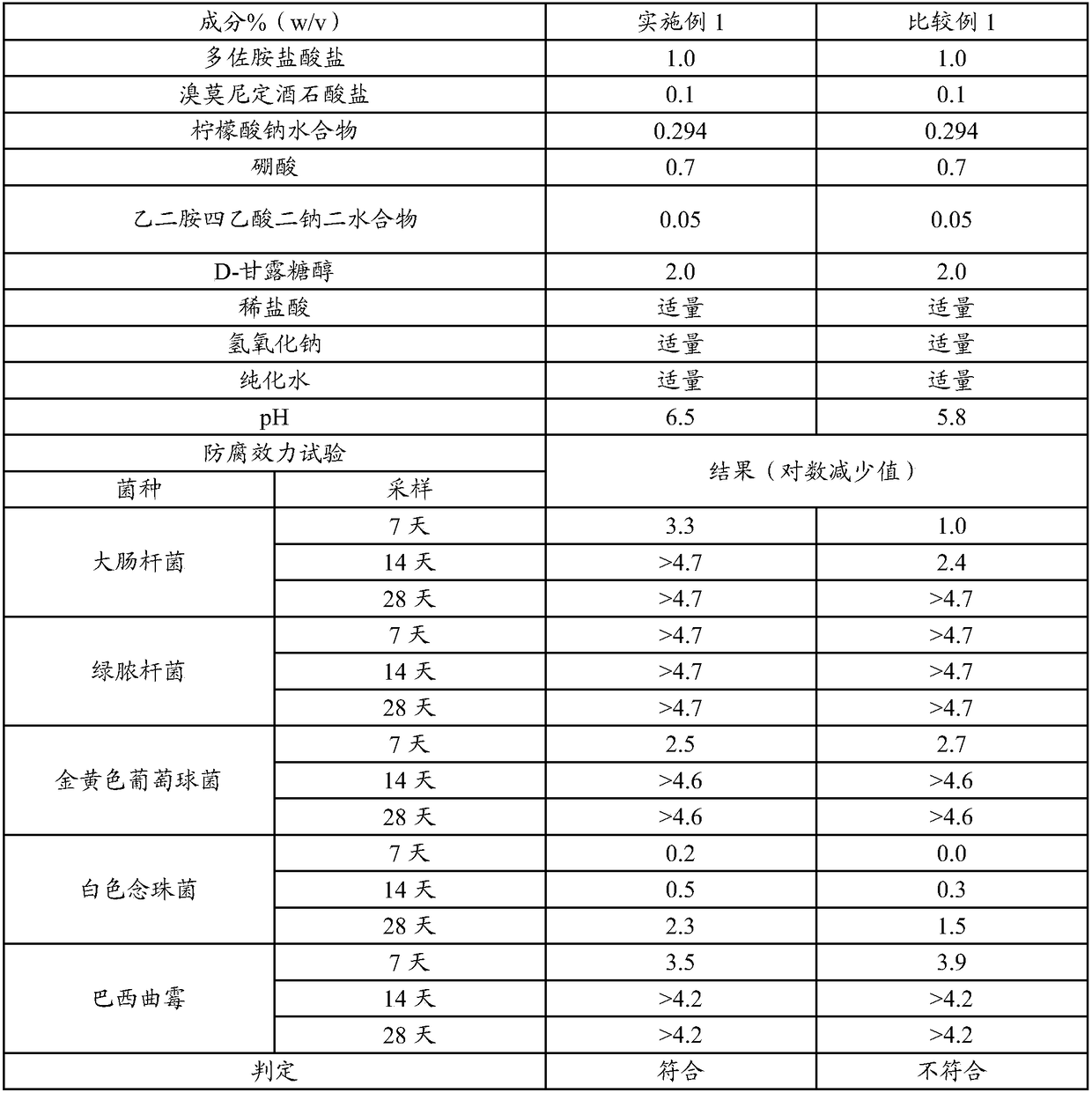
[0114] Anti-corrosion efficacy test (1)
[0115] 1. Preparation of test preparations
[0116] Dorzolamide hydrochloride (1g), brimonidine tartrate (0.1g), sodium citrate hydrate (0.294g), boric acid (0.7g), D-mannitol (2.0g), ethylene glycol Dissodium amine tetraacetate dihydrate (0.05 g) was dissolved in water and sterilized by filtration. After adjusting the resulting solution to pH 6.5 with a pH regulator, water was added to make the total amount 100 mL, thereby preparing the preparation of Example 1 . The preparation of Comparative Example 1 was prepared in the same manner as that of Example 1 except for changing the pH value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com