Compound contraceptive patch containing drospirenone and estrogen, preparation method and application
A drospirenone and compound technology, applied in the field of compound contraceptive patch and its preparation, can solve the problems of poor compliance, frequent use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
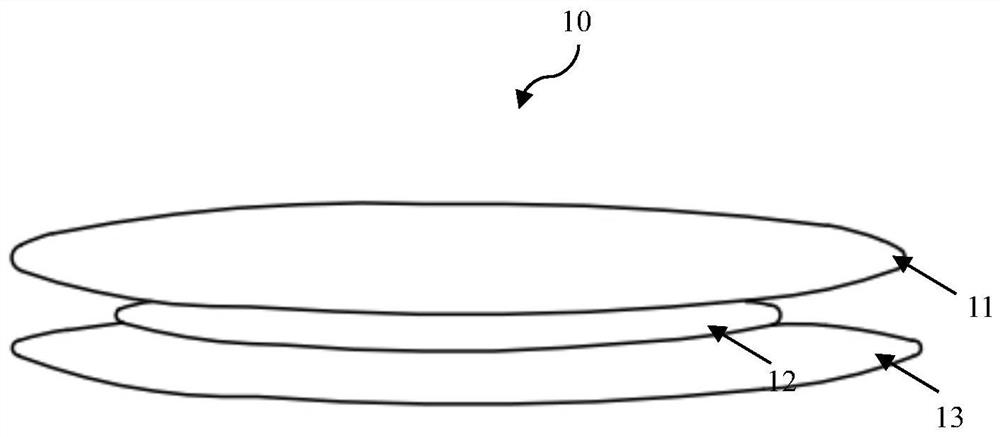
[0041] Such as figure 1 As shown, the compound contraceptive patch 10 is composed of a backing layer 11 , an anti-adhesive layer 13 and a drug-loaded layer 12 . The drug-loaded layer 12 is coated on the backing layer 11, the anti-adhesive layer 13 is covered on the drug-loaded layer 12, and the anti-adhesive layer 13 is peeled off during use. Ketones and estrogen act as a synergistic contraceptive. The specific formulation of the drug on the drug-loaded layer 12 is shown in Example 2-Example 5.
Embodiment 2
[0043] Prescription: drospirenone 0.3g, ethinylestradiol 6mg, azone 0.15g, polyacrylate pressure-sensitive adhesive 6g.
[0044] Preparation method: Add drospirenone and ethinyl estradiol to 4 g of ethyl acetate, stir to dissolve, and obtain a liquid medicine for later use. Add polyacrylate pressure-sensitive adhesive and azone into the drug solution, stir at 800 rpm / min for 30 minutes, and place to degas to obtain drug-containing glue. Apply the drug-containing glue on the release layer, dry at 50°C for 30 minutes, and cover the backing layer to obtain the product. The anti-adhesive layer adopts the 9744 type release film of the American 3M Company, and the backing layer adopts the 9680 type backing film of the American 3M Company. Prepared to contain drospirenone 1.14mg / cm 2 , ethinyl estradiol 0.023mg / cm 2 patch.
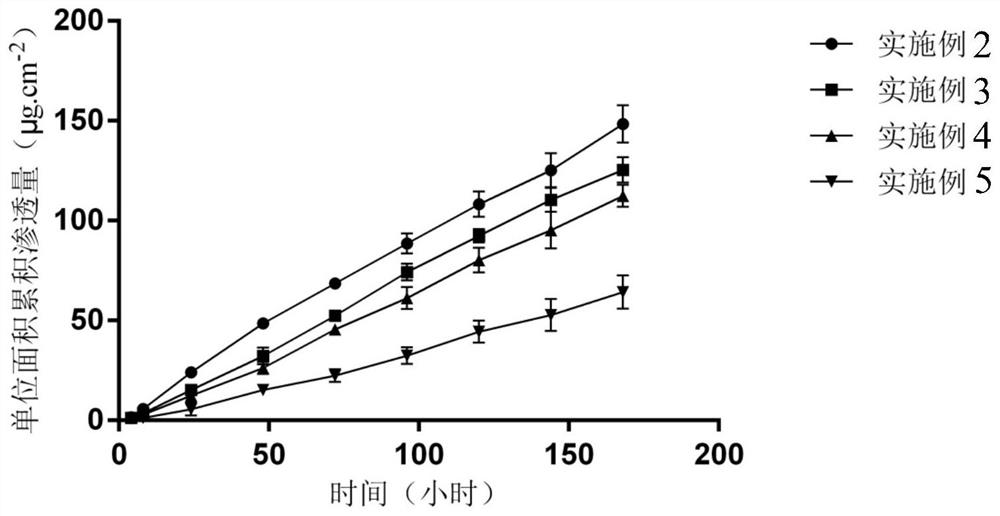
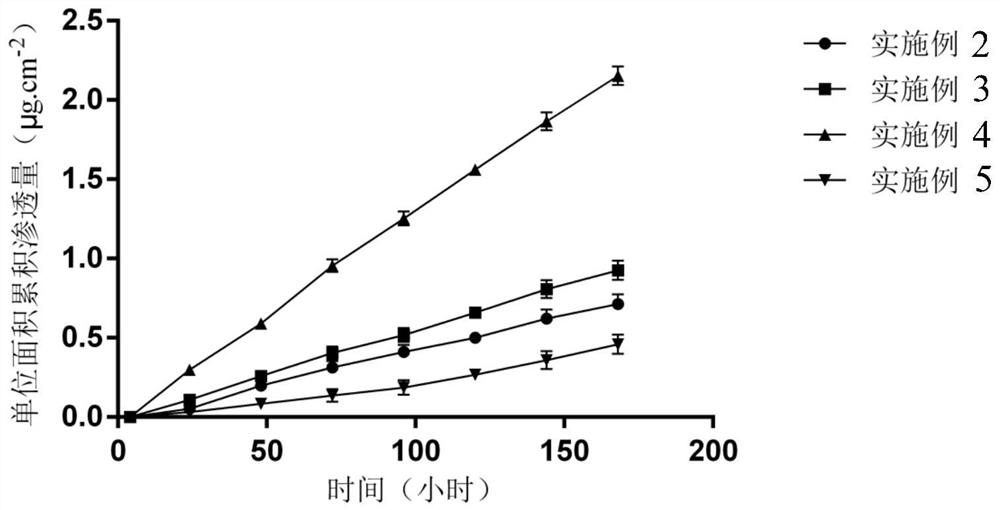
[0045]Measurement of permeation rate in vitro: Using a Franz vertical diffusion cell, the patch was attached to the horny layer of nude mouse skin, sandwiche...
Embodiment 3
[0047] Prescription: drospirenone 1g, ethinylestradiol 10mg, menthol 0.1g, isopropyl myristate, polyisobutylene pressure-sensitive adhesive 10g.
[0048] Preparation method: Add drospirenone and ethinyl estradiol to 8 g of acetone, stir to dissolve, and obtain a medicinal solution for later use. Add polyisobutylene pressure-sensitive adhesive, menthol and isopropyl myristate to the liquid medicine, stir at a speed of 600 rpm / min for 40 minutes, place to degas, and obtain the liquid glue containing the medicine. Apply the drug-containing glue on the release layer, dry at 70°C for 30 minutes, and cover the backing layer to obtain the product. The anti-adhesive layer adopts the 9748 type release film of the 3M Company of the United States, and the backing layer adopts the 9718 type backing film of the 3M Company of the United States. Prepared to contain drospirenone 0.73mg / cm 2 , ethinyl estradiol 0.042mg / cm 2 patch.
[0049] Measurement of permeation rate in vitro: Using a F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com