Polar cycloalkene copolymer and preparation method thereof
A cyclic olefin copolymer and copolymer technology, applied in the field of cyclic olefin copolymers, can solve the problems of low glass transition temperature and low molecular weight, and achieve good thermal and mechanical properties, good polarity and low water contact angle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
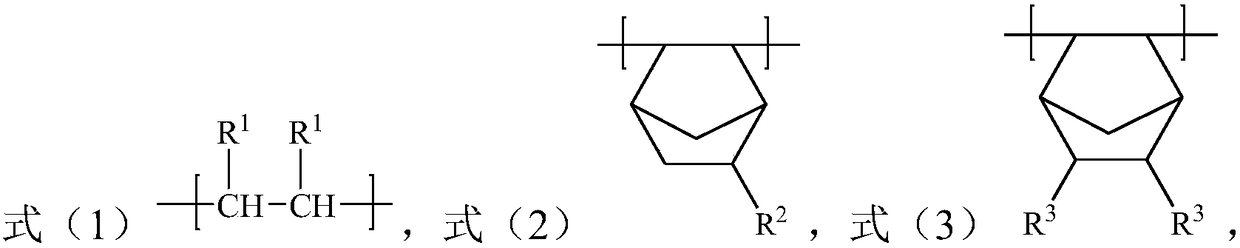
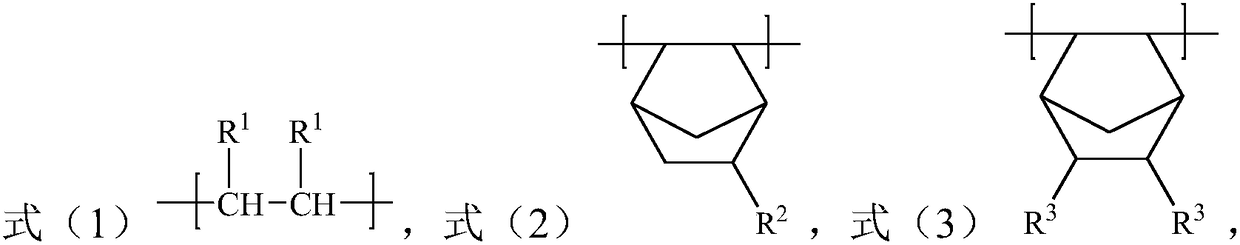
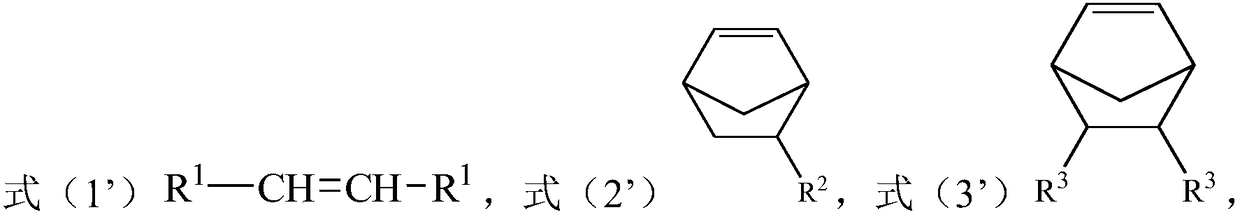
[0030] The present invention also provides a preparation method of a polar cycloolefin copolymer, the method comprising: in an organic solvent, in the presence of a catalyst, the compound represented by formula (1'), the compound represented by formula (2') Compound and compound A carry out solution polymerization reaction; Wherein, described compound A is the R in the compound shown in formula (3') 3 The compound after group is protected by protecting agent; In the obtained copolymer, take the total structural unit molar weight in the described copolymer as a basis, the content of the structural unit shown in formula (1) is 40-75mol%, formula (2 The content of the structural unit shown in ) is 20-40mol%, and the content of the structural unit shown in formula (3) is 5-30mol%;
[0031]
[0032] Among them, each R 1 each independently selected from H and C1-C10 alkyl; R 2 selected from H, a C1-C10 hydrocarbon group containing an unsaturated double bond or a C1-C10 alkyl gr...
preparation example 1
[0107] The preparation method of two (salicylaldehyde pentafluoroaniline) titanium dichloride comprises:
[0108] (1) In 50mL toluene solvent, under the condition of 120 ℃, carry out condensation reaction of salicylaldehyde and pentafluoroaniline according to the molar ratio of 1:1 (the total amount is 0.05mol) for 10h to prepare the catalyst ligand salicylaldehyde Pentafluoroaniline;
[0109] (2) Under anhydrous and oxygen-free conditions at room temperature (about 25°C), in 30mL of dichloromethane solvent, salicylaldehyde pentafluoroaniline and bis(tetrahydrofuran)titanium dichloride in a molar ratio of 2:1 (the total amount is 0.003 mol) complexation reaction was carried out for 12 hours to prepare the final target product bis(salicylaldehyde pentafluoroaniline)titanium dichloride.
preparation example 2
[0111] The preparation method of two (3,5-methyl salicylaldehyde pentafluoroaniline) titanium dichloride comprises:
[0112] (1) In 50mL of toluene solvent, under the condition of 120°C, condense 3,5-methyl salicylaldehyde and pentafluoroaniline according to the molar ratio of 1:1 (the total amount is 0.05mol) for 10h to prepare Catalyst ligand 3,5-methyl salicylaldehyde pentafluoroaniline;
[0113] (2) Under anhydrous and oxygen-free conditions at room temperature (about 25°C), in 30mL of dichloromethane solvent, 3,5-methyl salicylaldehyde pentafluoroaniline and bis(tetrahydrofuran)titanium dichloride are mixed according to moles The ratio of 2:1 (the total amount is 0.003 mol) was carried out for 12 hours for the complexation reaction to prepare the final target product bis(3,5-methylsalicylaldehyde pentafluoroaniline)titanium dichloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com