Preparation method of immunogenicity-enhanced porcine transmissible gastroenteritis S gene replication-defective recombinant adenovirus
A recombinant adenovirus and replication-deficient technology, applied in the field of biological vaccines, can solve the problems of low immunogenicity and inability to achieve immune effects, and achieve the effect of strong ability and prevention of TGE
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 Transformation of recombinant adenovirus shuttle plasmid pacAd5 CMV K-N pA
[0044] According to the ribosome binding site sequence of encephalomyocarditis virus published in the GenBank database (GenebankSequence ID: gb|EF591488.1), synthesize the IRES sequence, and add restriction sites EcoRI and BamHI at both ends of the sequence to obtain the plasmid pEx-IRES .
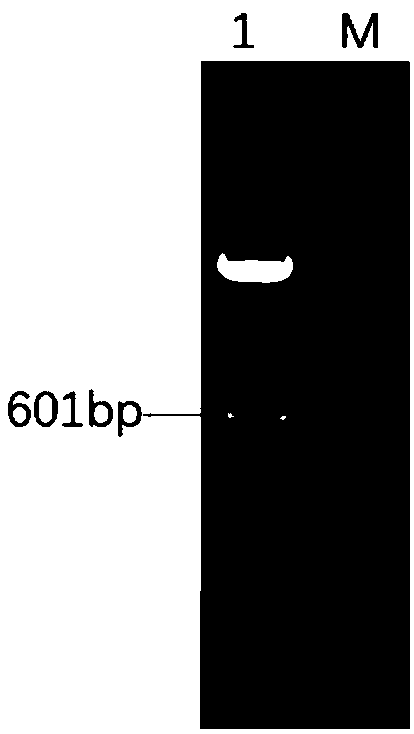
[0045] The plasmids pEx-IRES and pacAd5 CMV K-N pA were extracted respectively, and the plasmids were respectively digested with restriction endonucleases EcoRI and BamHI, and the double-digested IRES fragment and pacAd5 CMV K-N pA plasmid fragment were purified by agarose gel electrophoresis, and passed through T4 After ligase ligation, DH10b competent cells were transformed, and the shuttle plasmid pacAd5 CMV K-N pA-IRES containing IRES elements was identified and screened, such as figure 1 shown.
Embodiment 2
[0046] Example 2 Construction of recombinant adenovirus shuttle plasmid pacAd5 CMV K-N pA-sAD-IRES
[0047] 2.1 Design of specific primers P1, P2, P3, P4. According to the S gene sequence of TGEV Purdue 115 strain published in the GenBank database, two pairs of specific primers were designed, primers P1 and P2 were used to amplify the sequence of the A antigen epitope, and the length was 501bp; primers P3 and P4 were used to amplify the D antigen The sequence of the epitope. The primer sequences are as follows:
[0048] P1: GGGTACCATGTTAGTTACCAAAACAGCCGT
[0049] P2: AACTTGGGATCCTATTGTCCAGAAAAA
[0050] P3: TTTTCGGGATCCCAAGTTGAAAACACAG
[0051] P4: GGAATTCAAACTATTATCAGACGGT
[0052] 2.2 Cloning plasmid construction containing TGEV S gene. TGEV RNA was extracted using a viral RNA column extraction kit, and TGEV cDNA was obtained by reverse transcription using OligodT as a primer. Using TGEV cDNA as a template, P1 and P2 were used as primers to amplify the sequence sA cont...
Embodiment 3
[0054]Example 3 Construction of recombinant adenovirus shuttle plasmid pacAd5 CMV K-N pA-sAD-IRES-Ag85A
[0055] According to the Mycobacterium tuberculosis Ag85A gene published in the GenBank database, the Ag85A gene sequence (1364bp) was synthesized, and restriction sites SpeI and NotI were added at both ends of the sequence to obtain the plasmid pEx-Ag85A. According to the DNA sequence of the Ag85A gene, primers P5 and P6 were designed for the amplification of the Ag85A gene.
[0056] P5: 5'CTAGACTAGTATGCAGCTTGTTGACAGG3'
[0057] P6: 5'ATAAGAATGCGGCCGCCTAGGCGCCCTGGGGCGCGG3'
[0058] Utilize restriction endonuclease SpeI and NotI to digest plasmid pEx-Ag85A and the plasmid pacAd5 CMV K-N pA-sAD-IRES obtained in Example 2, after agarose gel purification and recovery, the Ag85A fragment is inserted into the digested Carrier pacAd5 CMV K-N pA-sAD-IRES, the ligation product was transformed into DH5α competent cells, positive clones were identified and screened by PCR, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com