Thrombopoietin fusion protein, preparation method and applications thereof
A technology of thrombopoietin and fusion protein, applied in the field of genetic engineering pharmaceuticals, can solve the problems of immaturity and unpredictable results, achieve long half-life and promote the effect of platelet production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
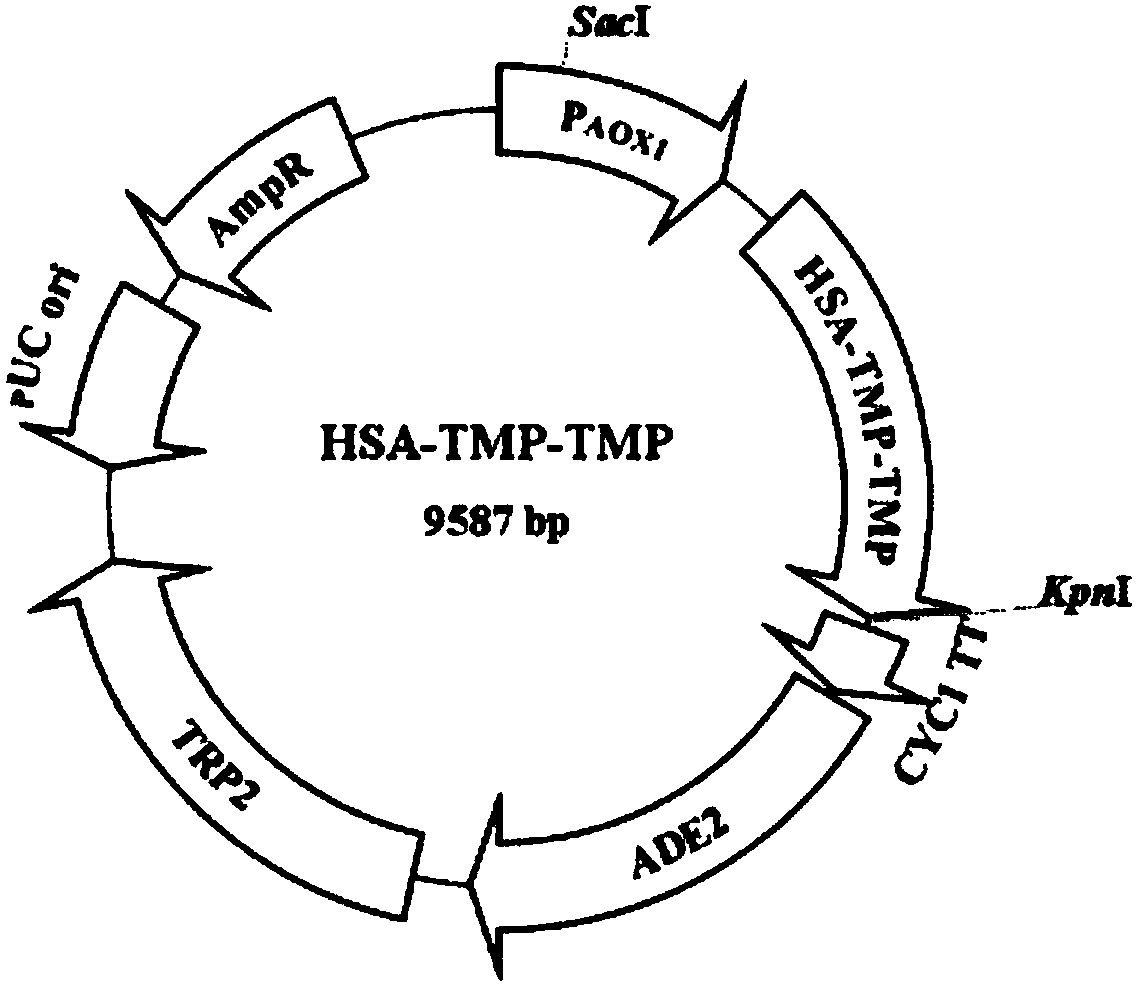
[0064] Example 1 Construction and expression of HSA-TMP fusion protein yeast expression vector
[0065] 1. Obtaining p29-simple-TMP sequence
[0066] 1. First encode according to the preferred codon pair of Pichia methanolophila (TMP) 2 The sequence of the gene is optimized.
[0067] 2. Entrust Dalian Takara Company to synthesize optimized (TMP) 2 Gene, (TMP) 2 The DNA sequence is as shown in SEQ ID NO:1, and loaded into p29-simple (p29-simple plasmid vector Dalian Takara company provides) to obtain the vector p29-simple-(TMP) 2 .
[0068] 3. The p29-simple-TMP already contains L, that is, the connecting peptide, the LDNA sequence is GGCGGCGGCGGTTCCGGACTGGAGCCCAAGAGCTGCGACAAGACCCACACCTGCCCTCCCTGCGAATTCGGTGGTGGCGGCAGC, and the amino acid sequence is GGGGSGLEPKSCDKTHTCPPCEF GGGGS.
[0069] 2. The cloning of HSA cDNA fragments:
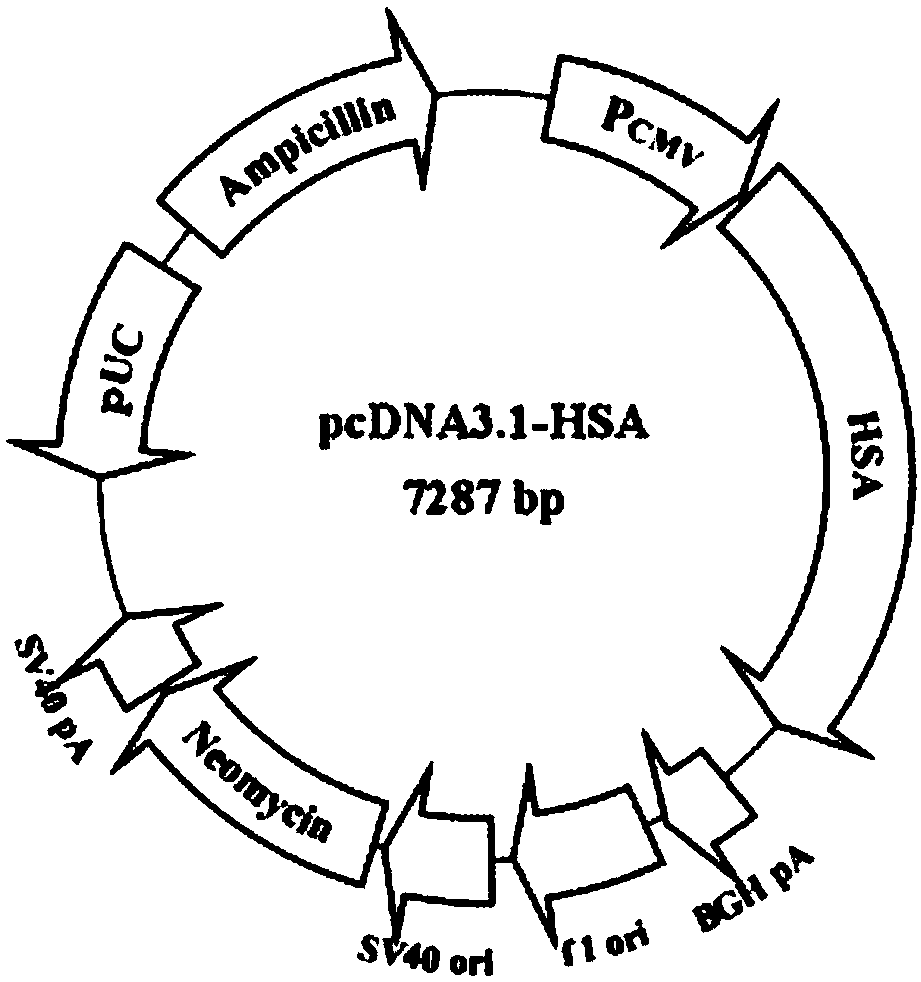
[0070] Obtained from the plasmid pcDNA3.1-fip-HSA clone (the HSA gene sequence was synthesized by the whole gene of Bao Biology, and the pcDNA3.1 plasmid was purch...
Embodiment 2
[0083] Example 2HSA-(TMP) 2 Biological activity detection of fusion protein
[0084] 1. Experimental process
[0085] Cells were plated, c-mpl, c-fos, pAdVAntage, Renilla, four plasmids were co-transfected. 48h after transfection, the positive control, blank control and test samples were added to detect the firefly luciferase activity and Renilla luciferase activity, and calculate the fluorescence ratio.
[0086] Positive control: TPIAO TPIAO (recombinant human thrombopoietin injection) purchased from Shenyang Sansheng Pharmaceutical Co., Ltd.
[0087] Blank control: serum
[0088] Fluorescence ratio = firefly fluorescence value / renilla fluorescence value.
[0089] For specific steps, see Chinese Patent (CN201310084212).
[0090] 2. Experimental results
[0091] Table 1HSA-(TMP) 2 Results of biological activity test of fusion protein
[0092] test group
Relative fluorescence ratio
Blank control group
1.00±0.02
Positive control group
1.44±0.12
Yeast strain 1-1
1.37±0.05
Yeas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com