Preparation and application of amphiphilic polypeptide genetic vector
A technology of gene carrier and polypeptide, applied in the field of nanomedical material preparation, can solve problems such as difficult degradation, and achieve the effects of safety toxicity, high cell transfection efficiency and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 500 mL THF and 1.0 g CaH 2 Added to a clean round bottom flask, stirred for 24 h and then distilled to obtain dry tetrahydrofuran. 500 mL of n-hexane and 1.0 g of CaH 2 Added to a clean round bottom flask, stirred for 24h and then distilled to obtain dry n-hexane. Mix 500 mL of DMF and 100 mL of toluene at 150 °C under N 2 Azeotropy occurs under protection, traces of water are evaporated, and when cooled to room temperature, dry DMF can be obtained.
[0034] (2) Preparation of CBZ-L-lysine-NCA monomer
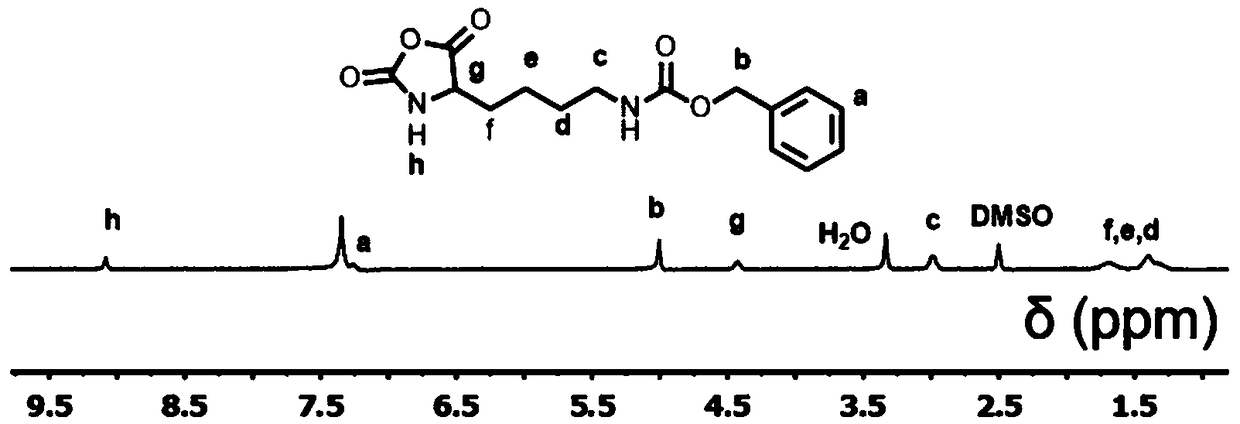
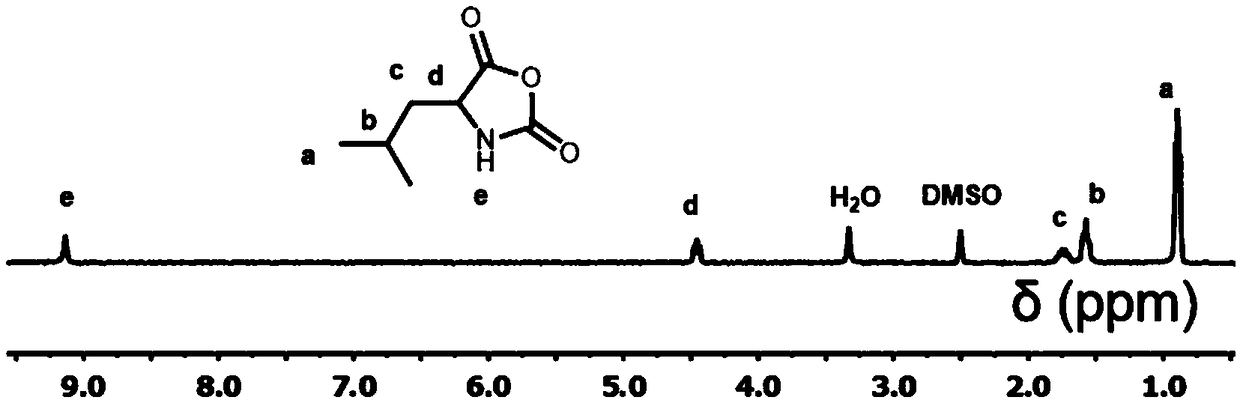
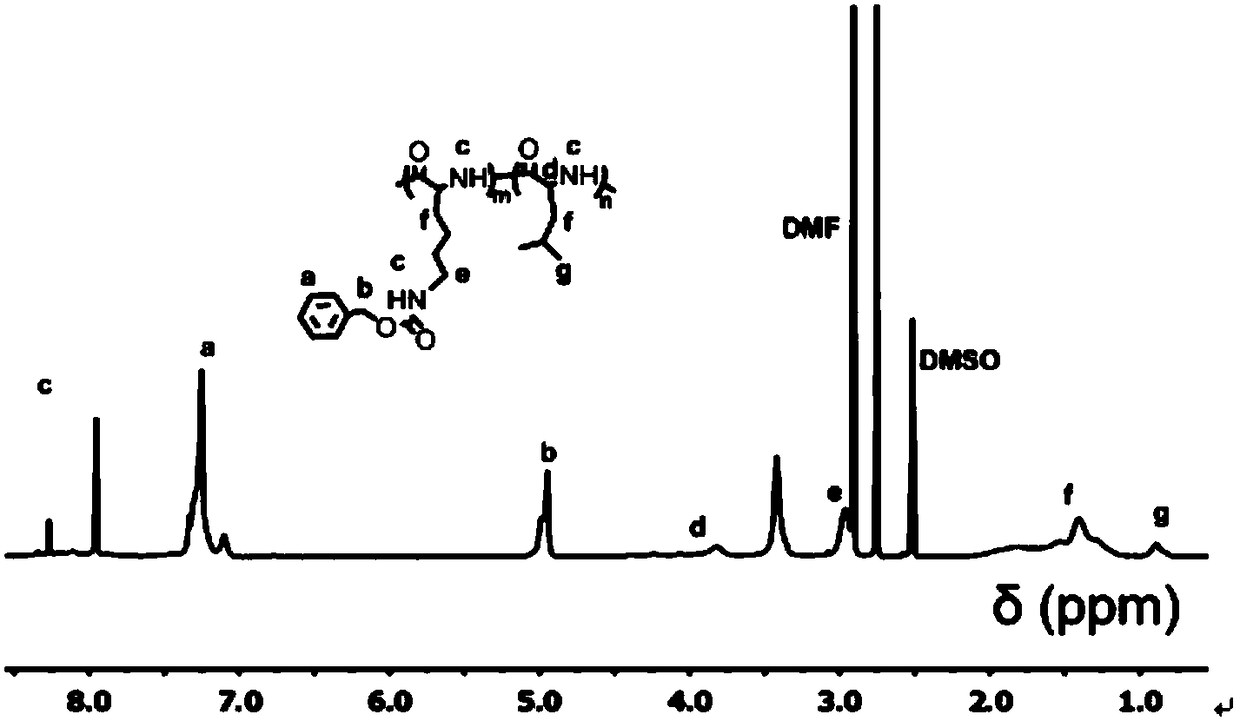
[0035] 5.0 g of N-carbobenzyloxylysine (purchased from Sigma-Aldrich Company) and 5.0 g of triphosgene were dissolved in dry 100 mL of tetrahydrofuran, and magnetically stirred at 50°C until the solution became clear and transparent. Then the solution was distilled and concentrated to 50 mL, and N-benzyloxycarbonyl (CBZ)-protected N-benzyloxycarbonyl-α-L-amino acid-N-carboxylic acid anhydride (CBZ-L-lysine) was obtained by precipitatio...
Embodiment 2
[0048] 500 mL THF and 1.0 g CaH 2 Added to a clean round bottom flask, stirred for 24 h and then distilled to obtain dry tetrahydrofuran. 500 mL of n-hexane and 1.0 g of CaH 2 Added to a clean round bottom flask, stirred for 24h and then distilled to obtain dry n-hexane. Mix 500 mL of DMF and 100 mL of toluene at 150 °C under N 2 Azeotropy occurs under protection, traces of water are evaporated, and when cooled to room temperature, dry DMF can be obtained.
[0049] (2) Preparation of CBZ-L-lysine-NCA monomer
[0050] 5.0 g of N-carbobenzyloxylysine (purchased from Sigma-Aldrich Company) and 5.0 g of triphosgene were dissolved in dry 100 mL of tetrahydrofuran, and magnetically stirred at 50°C until the solution became clear and transparent. Then the solution was distilled and concentrated to 50 mL, and precipitated with dry n-hexane to obtain the crude product of CBZ-L-lysine-NCA monomer. The crude product was then dissolved in 30 mL of tetr...
Embodiment 3
[0059] 500 mL THF and 1.0 g CaH 2 Added to a clean round bottom flask, stirred for 24 h and distilled to obtain dry THF. 500 mL of n-hexane and 1.0 g of CaH 2 Add it to a clean round bottom flask, stir for 24 h and distill to obtain dry n-hexane. Mix 500 mL of DMF and 100 mL of toluene at 150 °C under N 2 Azeotropy occurs under protection, traces of water are evaporated, and when cooled to room temperature, dry DMF can be obtained.
[0060](2) Preparation of CBZ-L-lysine-NCA monomer
[0061] 5.0 g of N-carbobenzyloxylysine (purchased from Sigma-Aldrich Company) and 5.0 g of triphosgene were dissolved in dry 100 mL of tetrahydrofuran, and magnetically stirred at 50°C until the solution became clear and transparent. Then the solution was distilled and concentrated to 50 mL, and precipitated with dry n-hexane to obtain the crude product of CBZ-L-lysine-NCA monomer. The crude product was then dissolved in 30 mL of tetrahydrofuran and recrystall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com