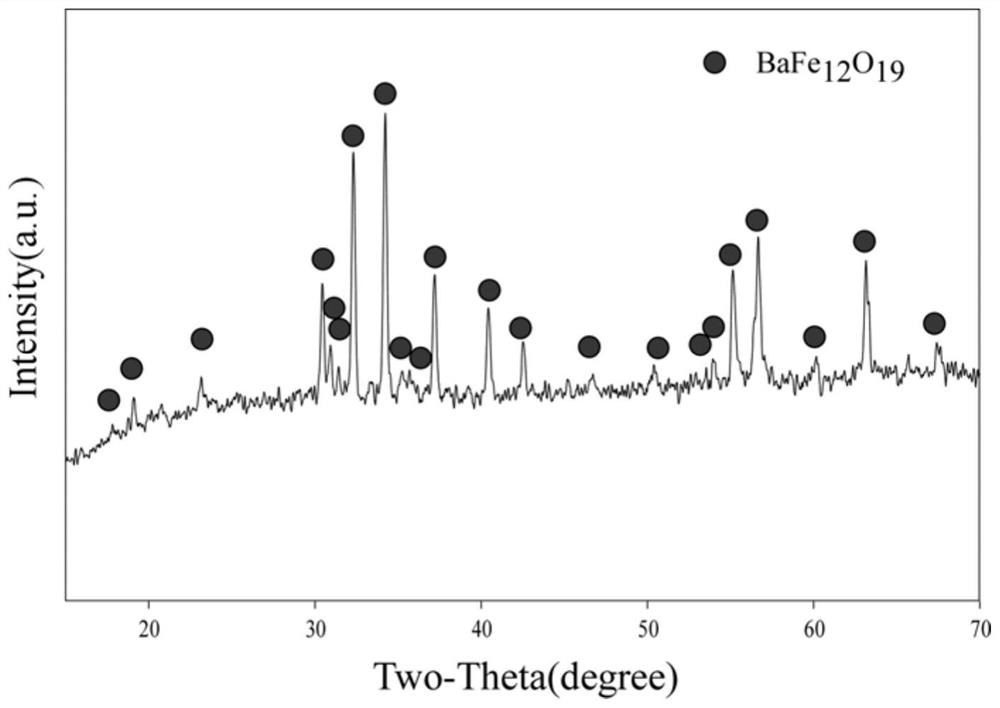
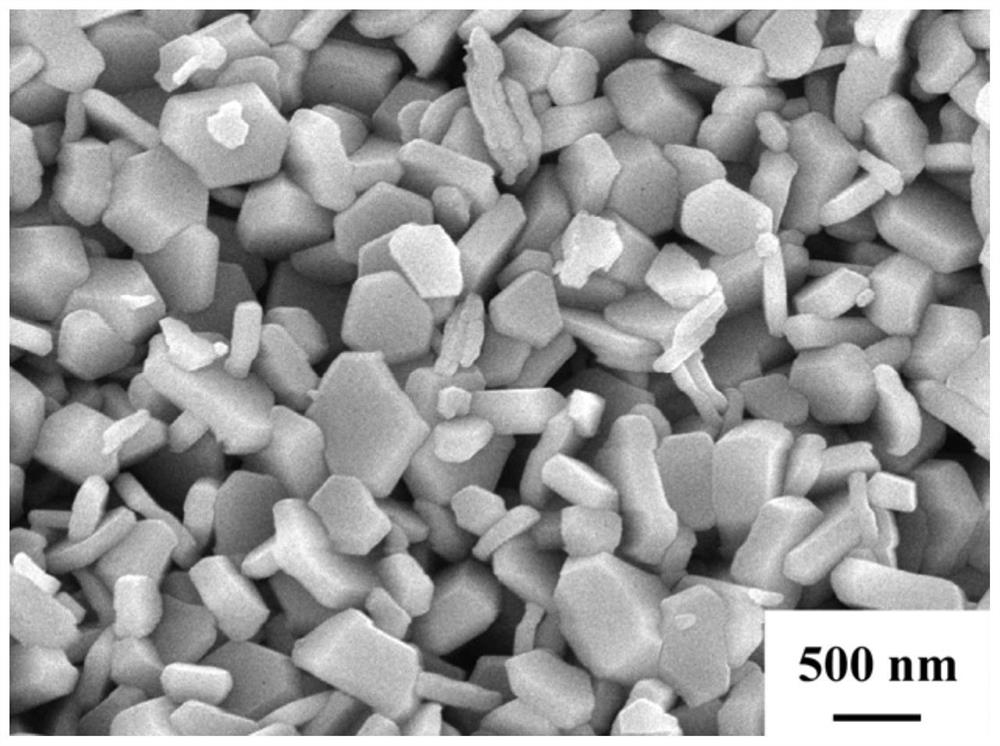
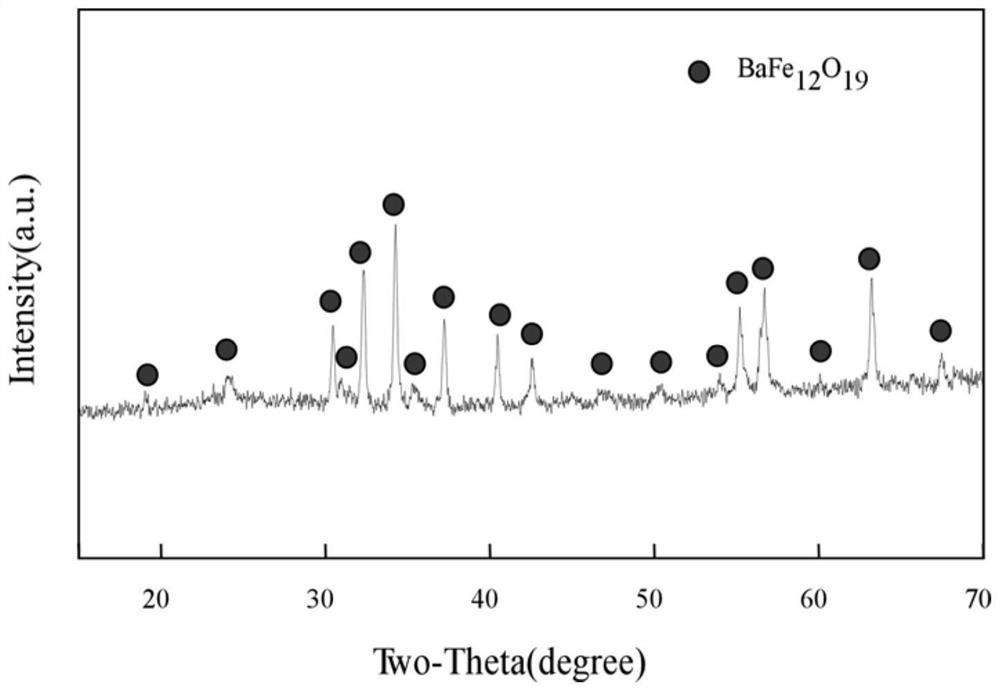
A kind of preparation method of high-purity hexagonal flaky barium ferrite
A technology of hexagonal flakes and barium ferrite, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of reducing the utilization rate of raw materials, environmental pollution, etc., and achieve improved utilization rate, uniform heating, and saving raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example provides a preparation process for preparing high-purity hexagonal flaky barium ferrite by chemical co-precipitation method. The preparation steps are as follows:
[0036] (1) Prepare BaCl with a concentration of 0.5mol / L 2 solution and 1mol / L FeCl 3 Solution, mix and stir evenly with barium iron stoichiometric ratio as 1:10; Stirring speed is 600 rev / min;
[0037] (2) Prepare a NaOH solution with a concentration of 6mol / L, and drop BaCl into it through a basic burette at a titration rate of 1 drop per second. 2 and FeCl 3 In the mixed solution, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0038] (3) Constantly stir with magnetic stirrer in the dropwise addition process, make reaction evenly, leave standstill 10 minutes after reaction finishes; Stirring speed is 600 rev / mins;
[0039] (4) Suction-filter and dry the precursor obtained from the reaction after mixing, and then heat up to 1000°C for 2 hours at a heating rate of 5°C / min;
...
Embodiment 2
[0046] This example provides a preparation process for preparing high-purity hexagonal flaky barium ferrite by chemical co-precipitation method. The preparation steps are as follows:
[0047] (1) Prepare BaCl with a concentration of 0.5mol / L 2 solution and 1mol / L FeCl 3 Solution, mix and stir evenly with barium-iron stoichiometric ratio as 1:9; Stirring speed is 600 rpm;
[0048] (2) Prepare a NaOH solution with a concentration of 6mol / L, and drop BaCl into it through a basic burette at a titration rate of 1 drop per second. 2 and FeCl 3 In the mixed solution, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0049] (3) Constantly stir with magnetic stirrer in the dropwise addition process, make reaction evenly, leave standstill 10 minutes after reaction finishes; Stirring speed is 600 rev / mins;
[0050] (4) Suction-filter and dry the precursor obtained by mixing and reacting, then heat up to 800°C at a heating rate of 5°C / min and calcinate for 2 hours;
[005...
Embodiment 3
[0053] This example provides a preparation process for preparing high-purity hexagonal flaky barium ferrite by chemical co-precipitation method. The preparation steps are as follows:
[0054] (1) Prepare BaCl with a concentration of 0.5mol / L 2 solution and 1mol / L FeCl 3 The solution is mixed and stirred evenly with the barium-iron stoichiometric ratio of 1:11; the stirring speed is 600 rpm;
[0055] (2) Prepare a NaOH solution with a concentration of 6mol / L, and drop BaCl into it through a basic burette at a titration rate of 1 drop per second. 2 and FeCl 3 In the mixed solution, BaCl 2 The stoichiometric ratio to the added NaOH is 1:38;
[0056] (3) Constantly stir with magnetic stirrer in the dropwise addition process, make reaction evenly, leave standstill 10 minutes after reaction finishes; Stirring speed is 600 rev / mins;
[0057] (4) Suction-filter and dry the precursor obtained from the reaction after mixing, and then heat up to 800°C at a heating rate of 5°C / min an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com