Preparation method for Y-type branched hydrophilic polymer carboxylic acid derivative
一种亲水性聚合物、羧酸衍生物的技术,应用在聚合物领域,能够解决产品分离困难、成本高、产率低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] The synthesis (prior art) of the polyethylene glycol-acetic acid (molecular weight 4000) of embodiment 1Y type branch
[0149]
[0150] With reference to the synthetic method of patent CN1243779C embodiment 5, the polyethylene glycol-acetic acid of the Y-type branch that molecular weight is 4000 is prepared: the polyethylene glycol monomethyl ether-aminoacetic acid (mPEG-Gly) that 10g molecular weight is 2000 and 10g molecular weight are 2000 polyethylene glycol monomethyl ether-succinimide carboxyacetate (mPEG-OCH 2 CO-NHS) was dissolved in 200ml of dichloromethane, 2.5ml of triethylamine was added to the solution, reacted overnight at room temperature, the solvent was concentrated by rotary evaporation, the residue was added with diethyl ether, the precipitate was collected by filtration, dried in vacuo, purified by ion exchange chromatography, gel Filter chromatography (GFC) monitoring starts to collect when the peak height of the target product exceeds 5mv, and e...
Embodiment 2
[0164] The synthesis (prior art) of the polyethylene glycol-acetic acid (molecular weight 20000) of embodiment 2Y type branch
[0165] With reference to the synthetic method of patent CN1243779C embodiment 5, the preparation molecular weight is the polyethylene glycol-acetic acid of the Y type branch of 20000: the polyethylene glycol monomethyl ether-aminoacetic acid (mPEG-Gly) that 10g molecular weight is 10000 and 10g molecular weight are 10000 polyethylene glycol monomethyl ether-succinimide carboxyacetate (mPEG-OCH 2 CO-NHS) was dissolved in 200ml of dichloromethane, 0.11ml of triethylamine was added to the solution, reacted overnight at room temperature, the solvent was concentrated by rotary evaporation, the residue was added with diethyl ether, the precipitate was collected by filtration, dried in vacuo, purified by ion exchange chromatography, and monitored by GFC , start to collect when the peak height of the target product exceeds 5mv, and end the collection when it ...
Embodiment 3
[0179] The synthetic (method of the present invention) of the polyethylene glycol-acetic acid (molecular weight 20000) of embodiment 3Y type branch
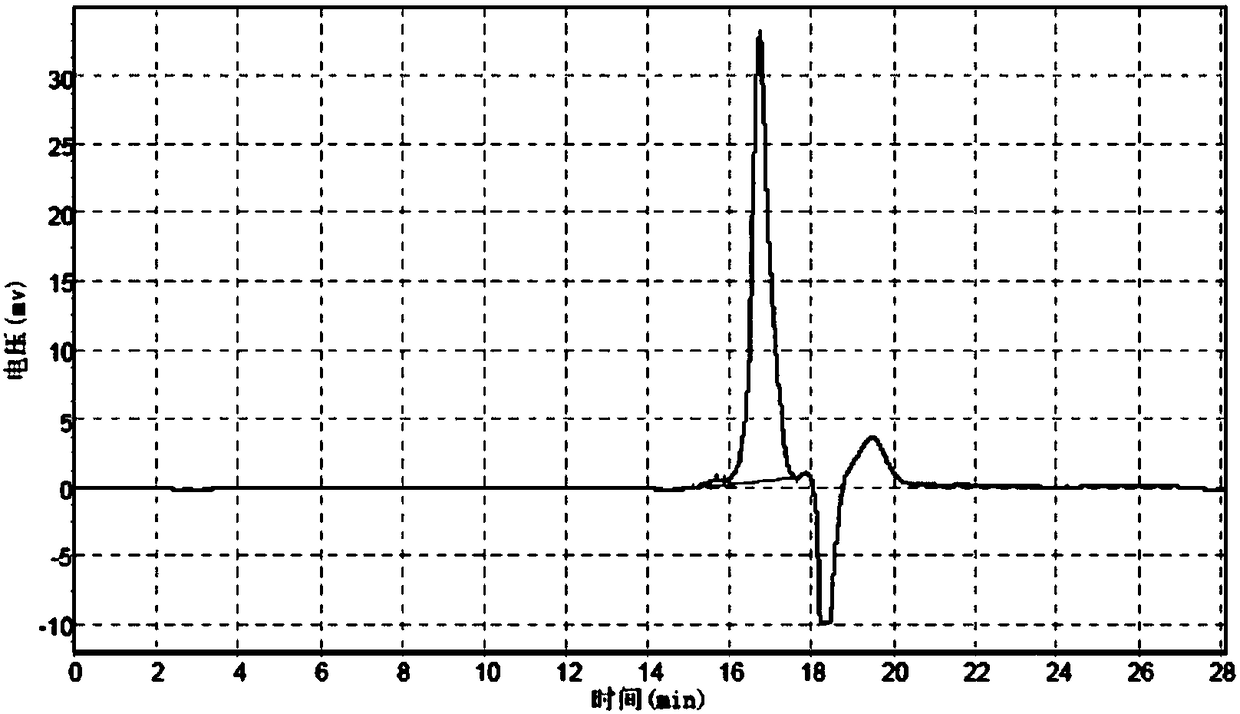
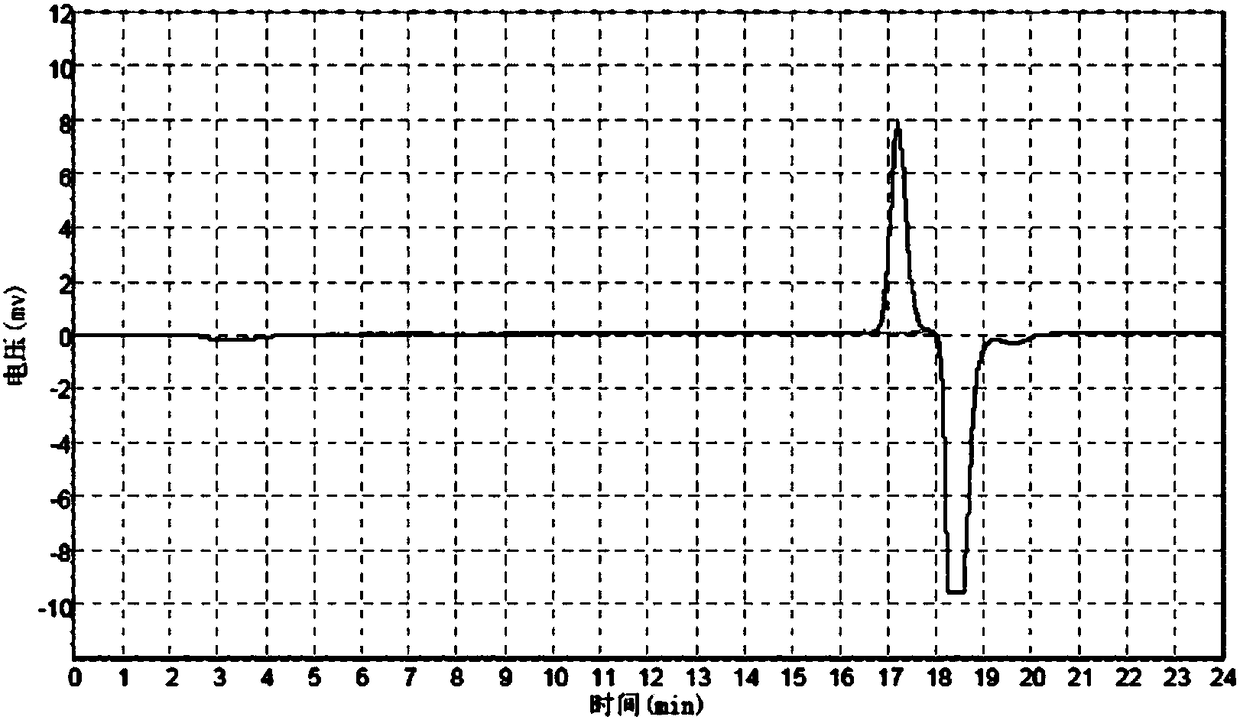
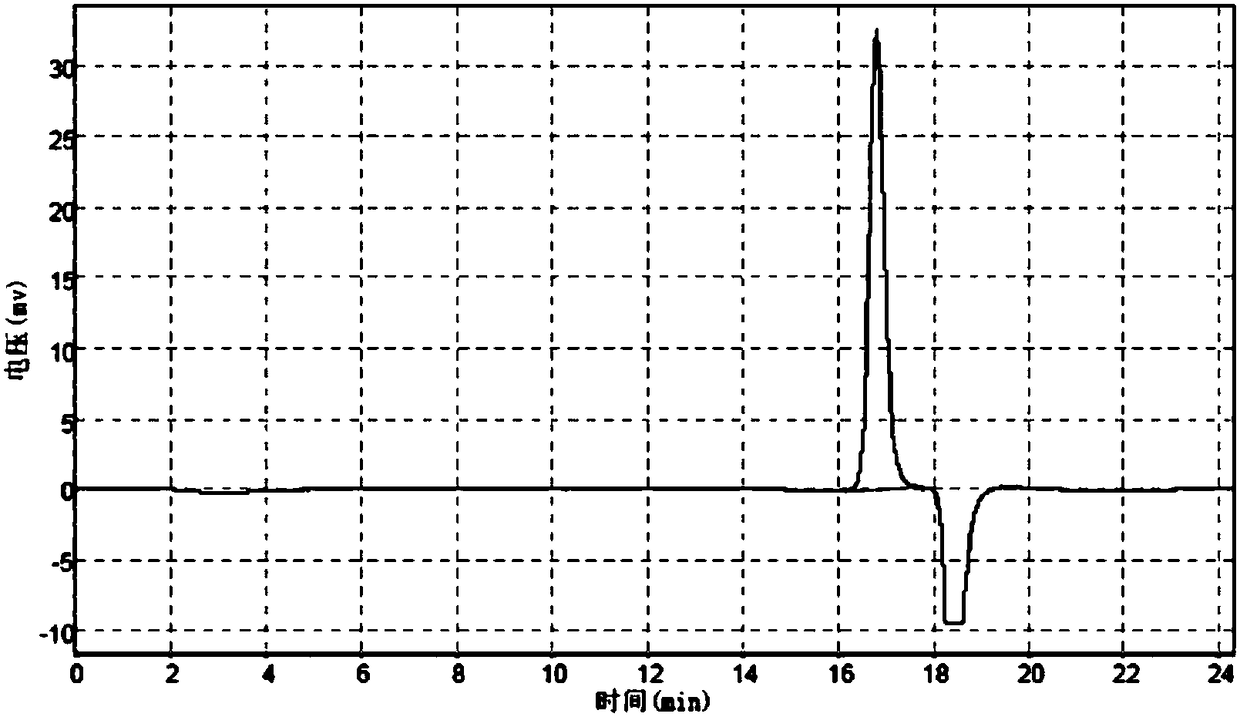
[0180] mPEG-Gly and mPEG-OCH 2 The steps of CO-NHS reaction refer to Example 2. After reacting overnight at room temperature, add BOC anhydride, react for 3 hours, concentrate the solvent by rotary evaporation, add diethyl ether to the residue, collect the precipitate by filtration, vacuum dry, purify by ion exchange chromatography, and monitor by GFC. The target product peak height exceeds 5mv to start collecting, and the collection ends when it is lower than 5mv.
[0181] The GFC chromatogram of the crude product before column separation is as follows: Figure 9 Shown, wherein the result analysis is shown in Table 9:
[0182] Table 9 Analysis result table
[0183]
[0184]
[0185] Collect the GFC chromatogram of the starting point as Figure 10 As shown, wherein the result analysis is shown in Table 10:
[0186] Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com