CH2 structure domain mutant of human IgG antibody Fc segment as well as preparation method and application thereof
A technology of mutants and structural domains, which is applied in the field of human IgG antibody Fc fragment CH2 domain mutants and preparations, can solve the problems of weak anti-aggregation ability and poor stability of Fc fragments, and achieve good stability, good anti-aggregation ability, The effect of reducing the risk of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Construction of the C-terminal phage display library of CH2
[0041] According to the CH2 gene sequence of IgG (GenBank: AAC82527.1), analyze its amino acid sequence, design the forward and reverse primers of the CH2 gene to amplify the target fragment (the horizontal line marks the Sfi I restriction site):
[0042] Forward primer (5`end to 3`end):
[0043] GT G GCC CAG GCGGCC GCACCTGAA CTC CTGGGGGGA CCG TCA GTCTTC CTCTTC-
[0044] Reverse primer (5`end to 3`end):
[0045]
[0046] The amplified fragment was digested with SfiI and ligated into the vector pComb3xSS to construct a phage display library.
Embodiment 2
[0047] Embodiment 2: candidate clone screening
[0048] 1. Panning experiment
[0049] 1) Take 5 tubes of TG1 bacteria, 2ml each. Shake to OD in about 4 hours 600 = 0.6.
[0050] 2) The phage library was first heated at 80°C for 10 minutes, and then left at room temperature for 20 minutes.
[0051] 3) Closed Panning orifice plate
[0052] a. Aspirate the coated protein and wash the plate once with PBS.
[0053] b. Add phage to BSA wells, 10 wells in 5 wells 12The final concentration of milk was 2%, 100 μl per well. Add 3% milk to anti-CH2 wells, 200μl per well.
[0054] c. Block for 1 hour at 37°C.
[0055] 4) combine
[0056] BSA well phages were transferred to anti-CH2 wells, 2h, 37°C.
[0057] 5) Elution
[0058] The phage liquid was discarded, and washed 10 times with PBST (5 times for each round, 4 rounds of screening in total).
[0059] 6) Infection
[0060] In the ultra-clean bench, add 100 μl of TG1 bacterial solution to each well of the Panning orifice pl...
Embodiment 3
[0083] Example 3: CH2 and KIKK molecular conformation and existing form (monomer, dimer, etc.)
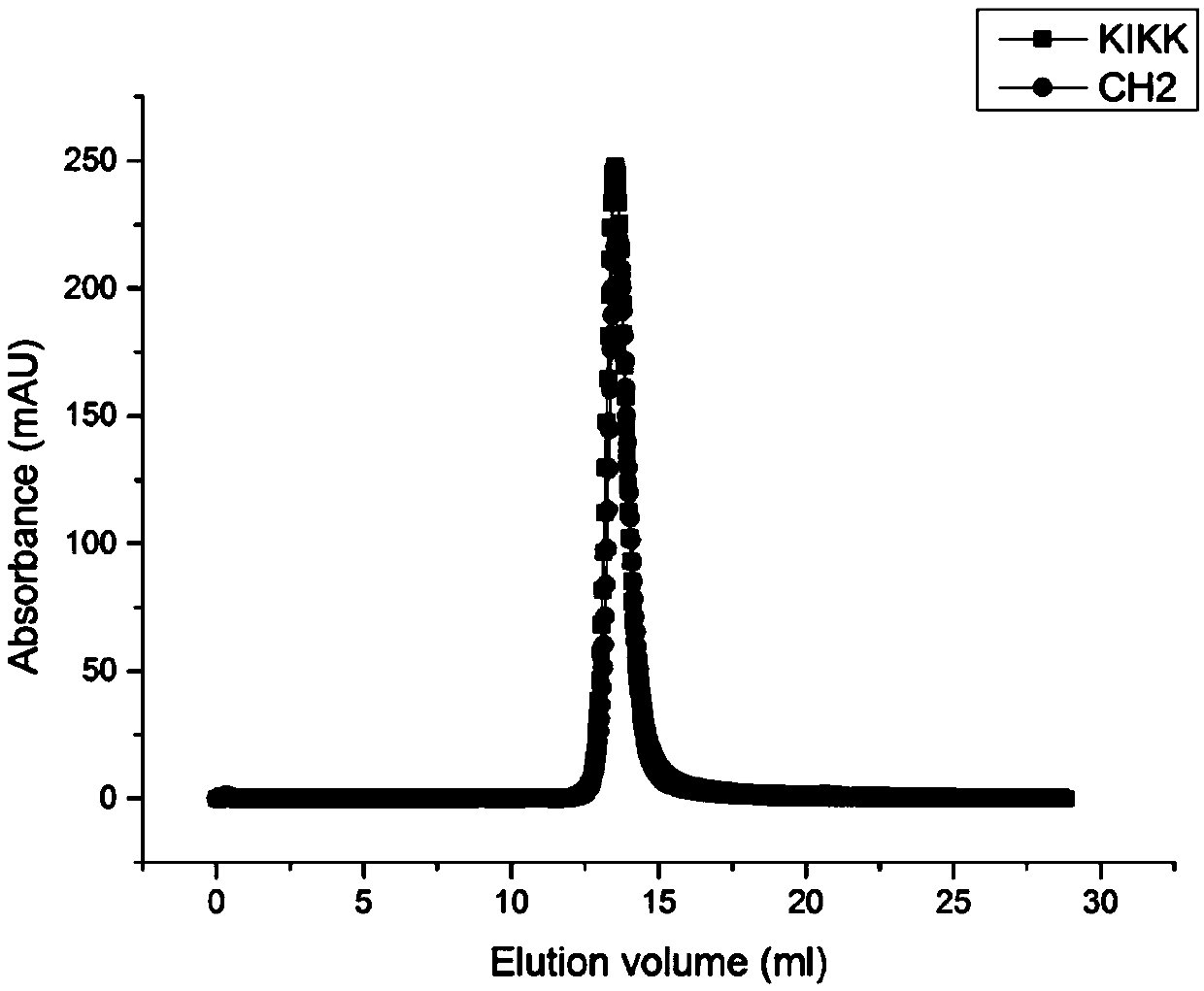
[0084] AKTA analysis of the existing forms of CH2 and KIKK: Concentrate the purified CH2 and KIKK proteins to 1 mg / ml, use PBS (pH7.4) as the elution buffer, pass through Column Superdex 75Increase 10 / 300GL, and the flow rate is 1ml / min, Then detect its existence form, such as figure 1 , compared with the standard curve, it can be found that the molecular weight of the two proteins is about 14kDa, and the results show that they exist in the form of monomers.
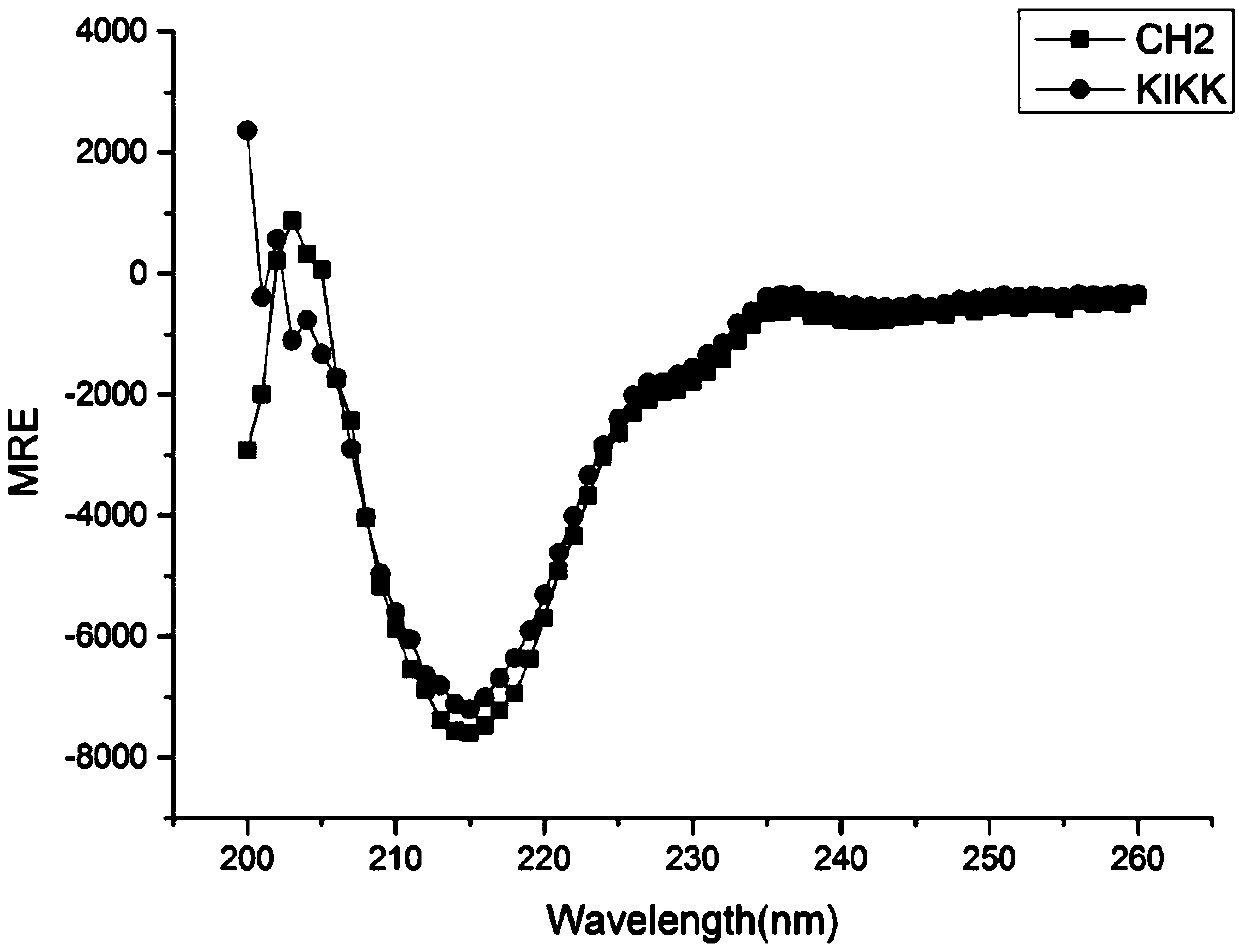
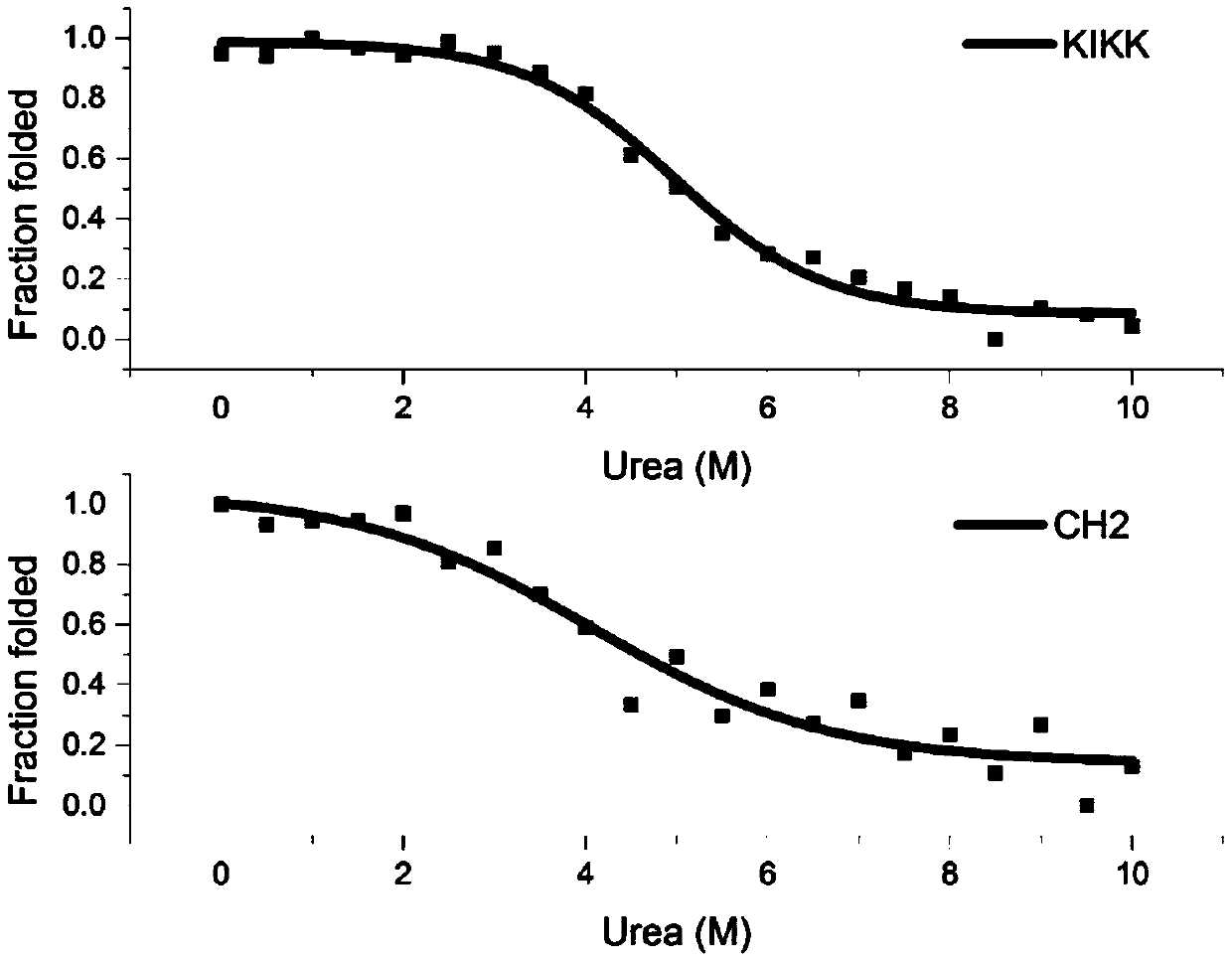
[0085] CD detection of protein molecular conformation: Dilute CH2 and KIKK proteins to 0.3 mg / ml, PBS (pH7.4) as a control, and detect their circular dichroism under different wavelength conditions at the near ultraviolet end (wavelength λ=190nm-260nm). Then analyze the protein secondary structure. like figure 2 As shown, there is an obvious trough at λ=218nm, indicating that the secondary structure of the above protein i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com