Testing method for thermal conductivity of phase change energy storage material
A technology of phase-change energy storage materials and testing methods, which is applied in the field of phase-change energy storage materials, can solve problems such as dehydration, difficulty in accurate measurement, and difficulty in preparing samples to be tested, so as to ensure stability and reliability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The purpose of this embodiment is to measure the CaCl in the cooling crystallization process. 2 ·6H 2 O at 29.2°C (i.e. the temperature to be measured T 0 ) at the thermal conductivity.
[0084] Specifically, the following steps are used for determination:
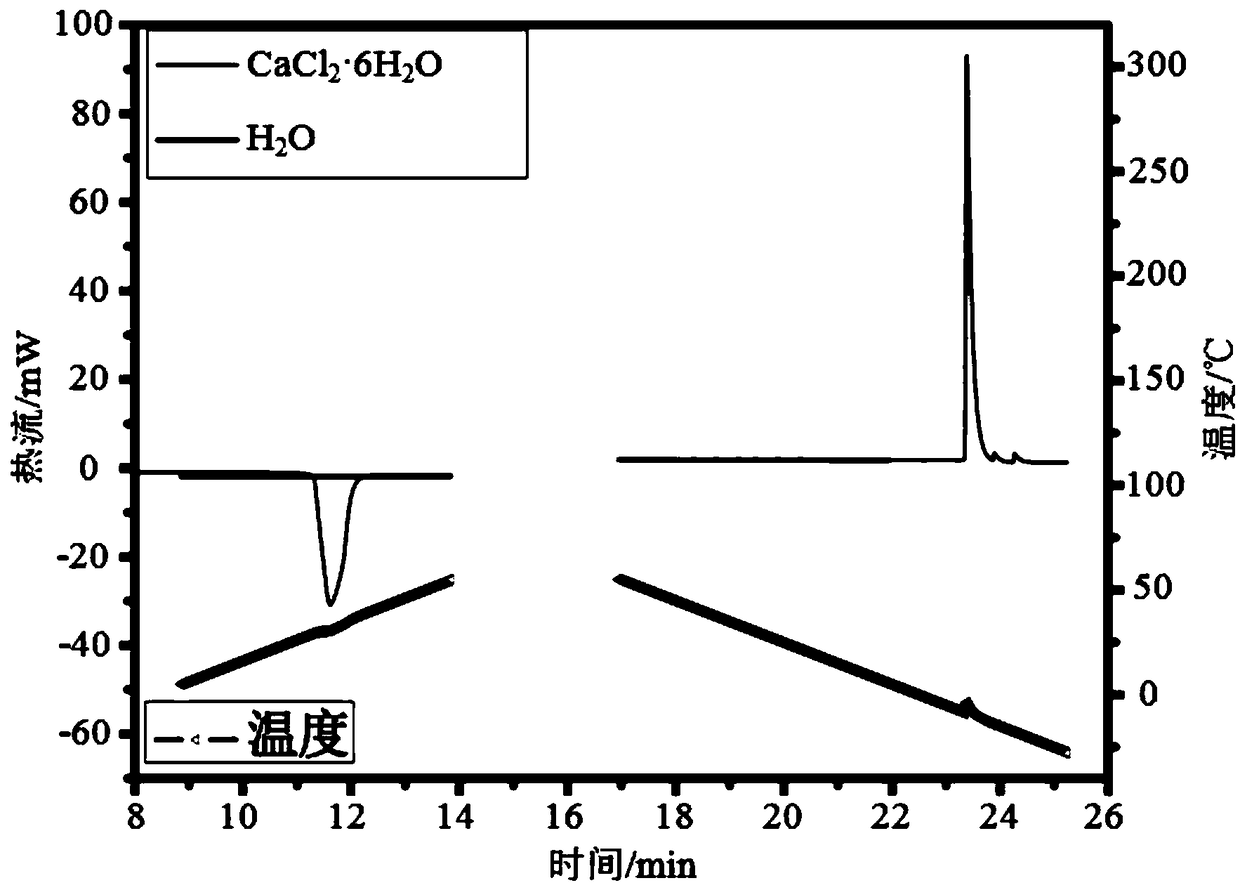
[0085] First, use DSC to test the empty sample pan (i.e. with a blank crucible as the background), water (m(H 2 O)=5.705mg) and CaCl 2 ·6H 2 O(m(Sam)=4.190mg) heat flow P(Bla), P(H 2 O) and P(Sam), the cooling rate is 10°C / min; the heat flow test results are as follows image 3 shown.
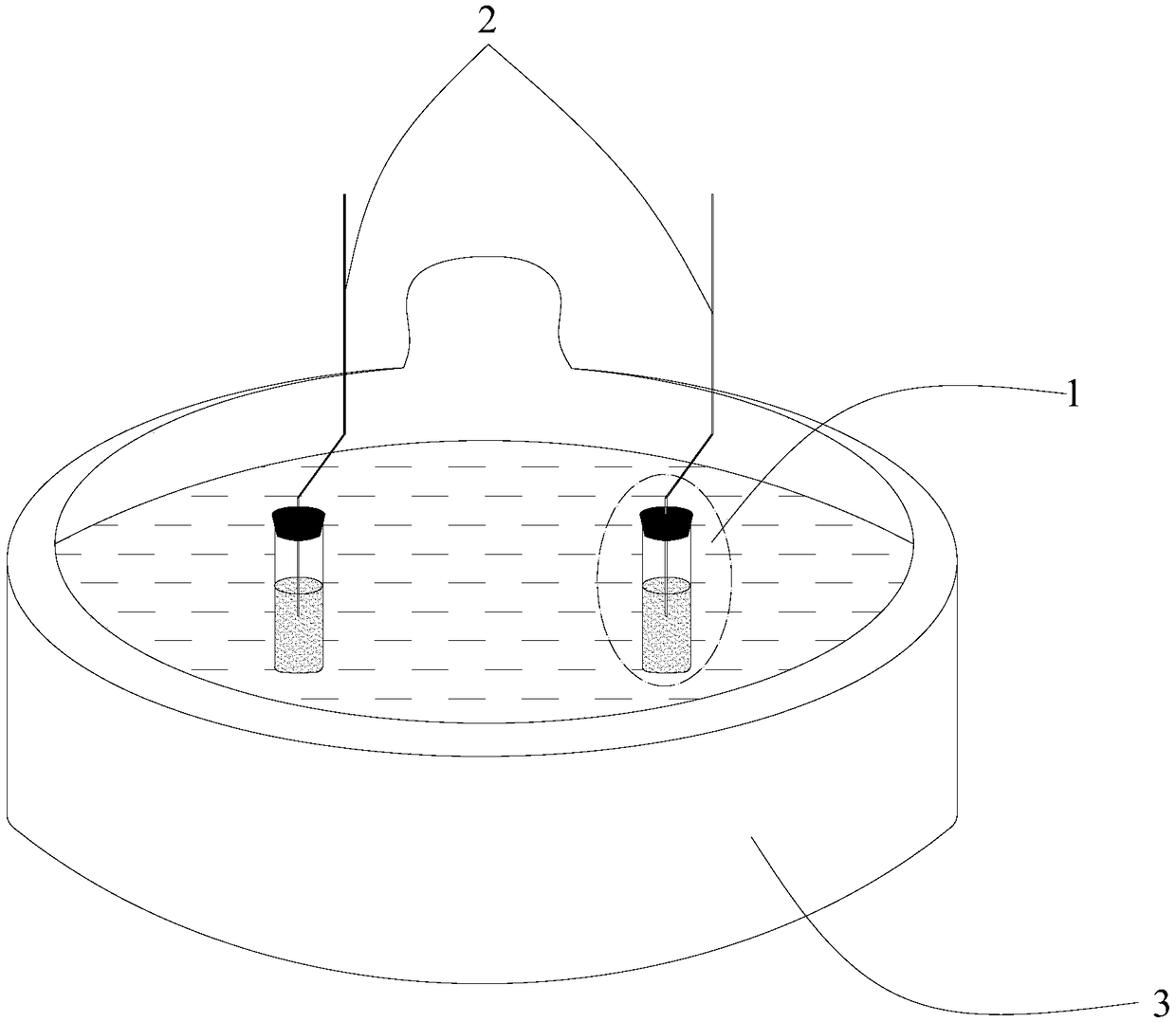
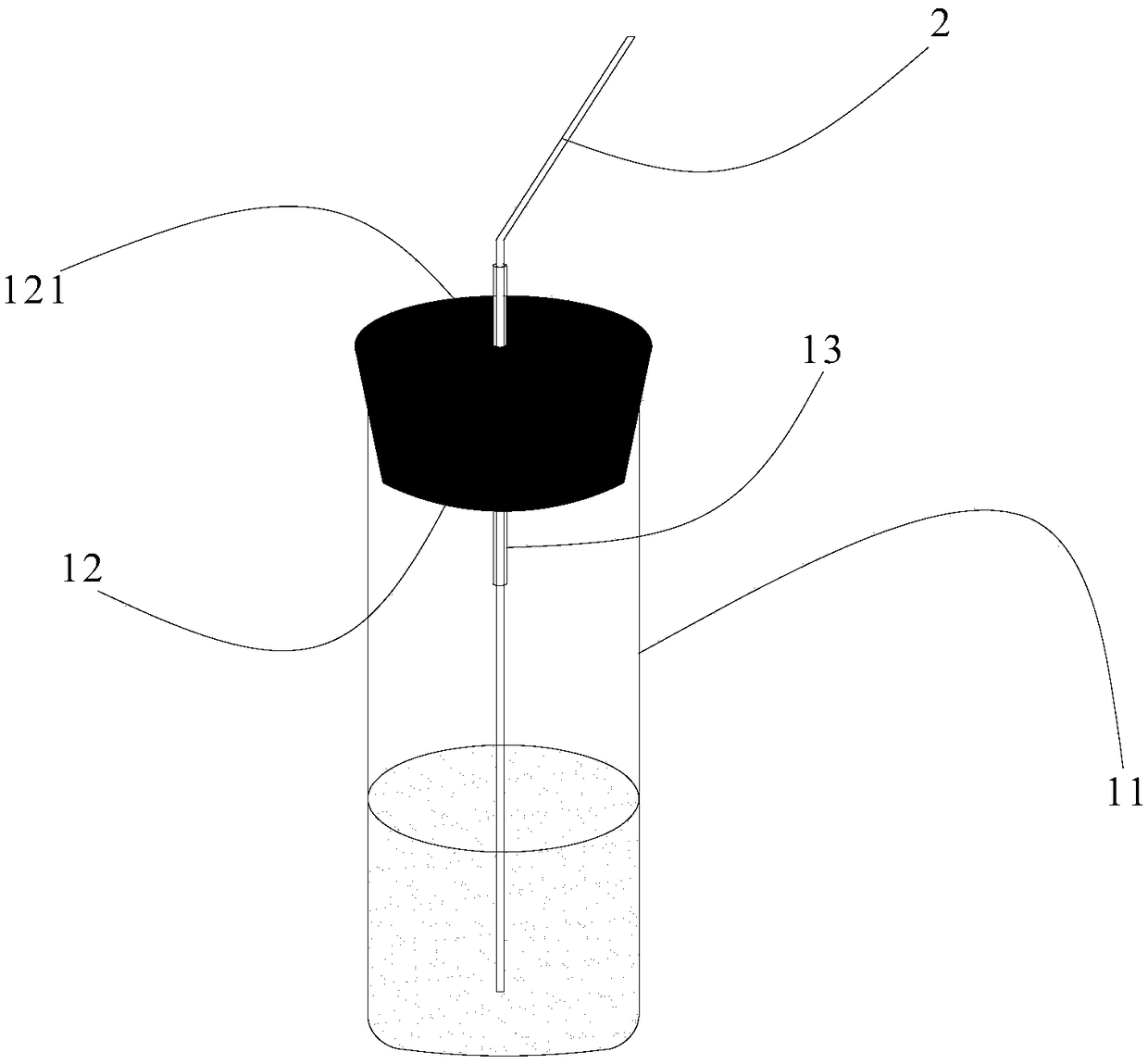
[0086] Then, 80mL of water (m'(H 2 O)=80.02g) and CaCl 2 ·6H 2 O(m'(Sam)=119.05g) were placed in figure 2 In the shown test tube 11, the test tube 11 is first placed in an ice-water bath, and shaken to make it crystallize at a temperature greater than 0°C (ice-water bath), CaCl 2 ·6H 2 Immediately after the O started to crystallize it was transferred to a 25 °C figure 1 In the water bath shown, and keep the water level...
Embodiment 2
[0091] The purpose of this embodiment is to measure the CaCl in the heating and melting process. 2 ·6H 2 O at 28.9°C (i.e. the temperature to be measured T 0 ) at the thermal conductivity.
[0092] Specifically, the following steps are used for determination:
[0093] First, use DSC to test the empty sample pan (i.e. with a blank crucible as the background), water (m(H 2 O)=5.705mg) and CaCl 2 ·6H 2 O(m(Sam)=4.190mg) heat flow P(Bla), P(H) heated between 30°C and 35°C 2 O) and P(Sam), the heating rate is 10°C / min; the heat flow test results are as follows image 3 shown.
[0094] Then, 80mL of water (m'(H 2 O)=80.02g) and CaCl 2 ·6H 2 O(m'(Sam)=119.05g) were placed in figure 2 In the test tube 11 shown, put the test tube 11 in a temperature of 35°C figure 1 In the water bath shown, and keep the water level line of the water bath and the water and CaCl in the test tube 2 ·6H 2 The surface of O is flat, water and CaCl are measured 2 ·6H 2 The temperature rise cu...
Embodiment 3
[0102] The purpose of this embodiment is to measure the CaCl in the heating process 2 ·6H 2 O liquid at 32.3 ℃ (that is, the temperature T to be measured 0 ) at the thermal conductivity.
[0103] Specifically, the following steps are used for determination:
[0104] First, use DSC to test the empty sample pan (i.e. with a blank crucible as the background), water (m(H 2 O)=5.705mg) and CaCl 2 ·6H 2 O(m(Sam)=7.590mg) heat flow P(Bla), P(H) heated between 29.5℃~35.0℃ 2 O) and P(Sam), the heating rate is 5°C / min; the heat flow test results are as follows Figure 5 and Image 6 shown. from Figure 5 and Image 6 It can be known that P(H 2 O)-P(Bla)=-1.863mW, P(Sam)-P(Bla)=-1.411mW; and it can be known from Example 2 that CaCl 2 ·6H 2 The average phase transition temperature of O in the heating process is 28.95°C, thus, the temperature T to be measured in this embodiment 0 With the sample to be tested CaCl 2 ·6H 2 The phase transition temperature T(Sam) of O is incon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal conductivity | aaaaa | aaaaa |
| Thermal conductivity | aaaaa | aaaaa |
| Thermal conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com