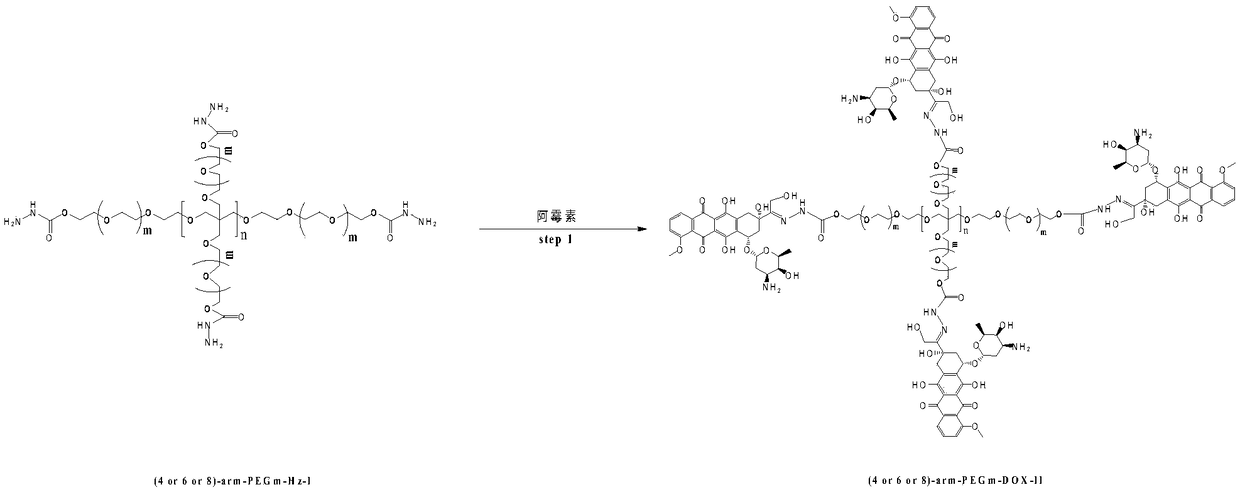
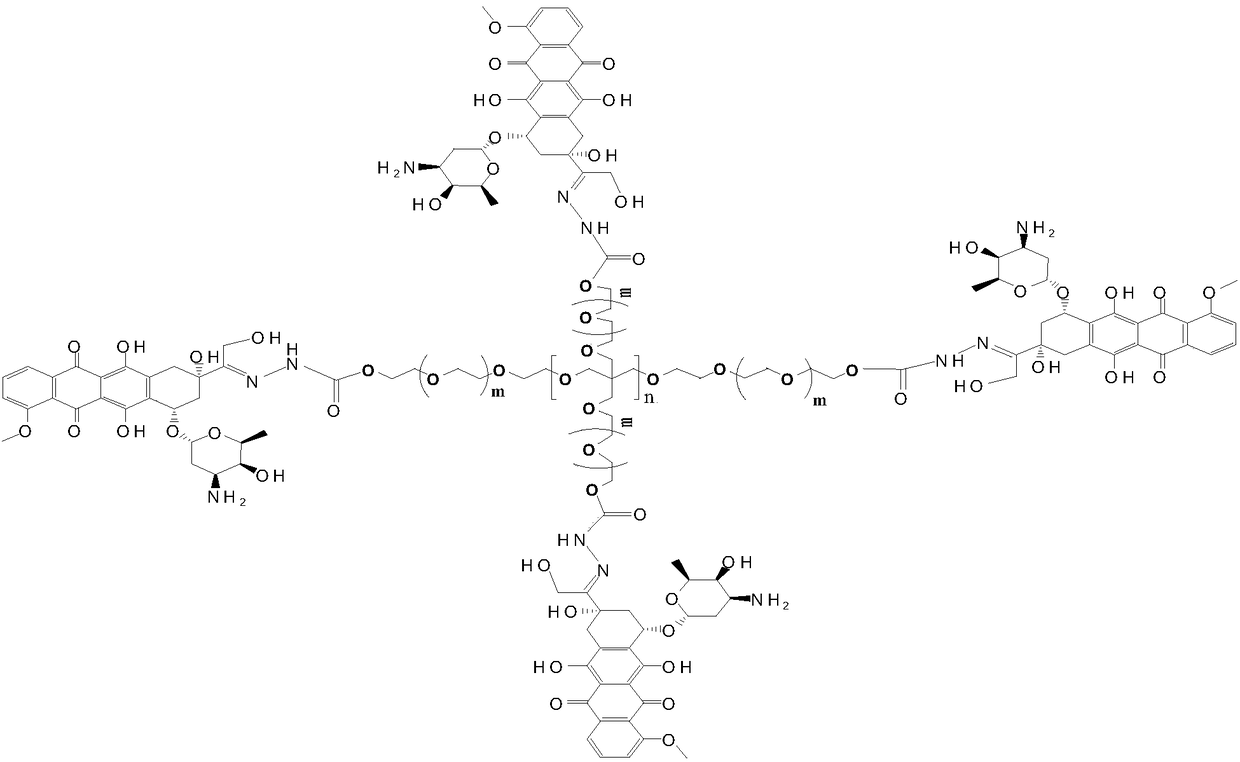
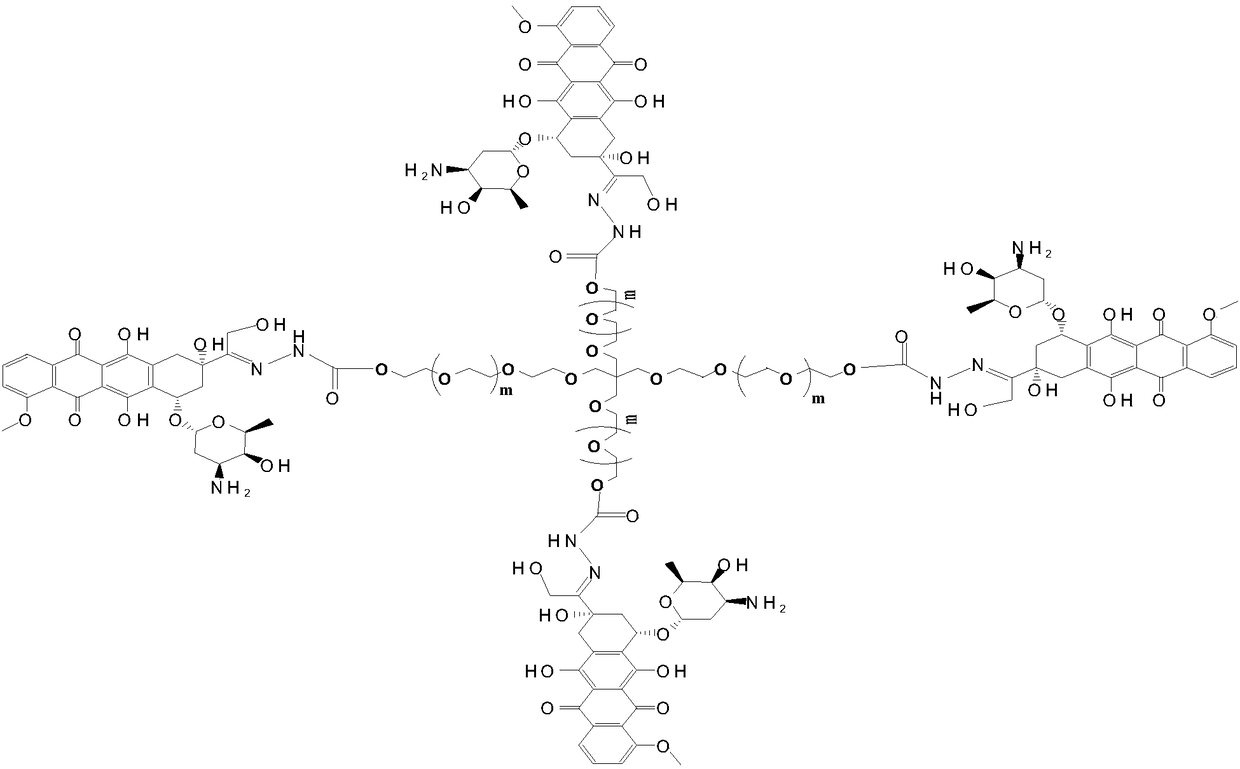
Multi-arm PEGylated (Polyethylene Glycolylated) azithromycin derivative and preparation thereof
A derivative and integer technology, used in the preparation of antitumor drugs, multi-arm PEGylated DOX derivatives and their preparation and preparation fields, can solve the toxicity and side effects of tissues and organs, limited drug immobilization rate, and drug The slow release effect is not obvious and other problems, so as to reduce the toxic and side effects, increase the existence time, and reduce the toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 4arm-PEG24-DOX(III)
[0029] 10 mmol equivalent of 4-arm-PEGm-Hz-I and 6 mmol of doxorubicin hydrochloride were dissolved in anhydrous DMF solvent, and 1 mmol% catalytic equivalent of phosphoric acid was added to the organic solvent. The reaction was carried out in the dark for 48 hours at a reaction temperature of 10°C, and the target product was obtained by recrystallization through column chromatography after the completion of the detection reaction. Yield: 83%. The NMR data are as follows: 1 H NMR (400MHz,DMSO)δ,9.05(s,1H),8.89–8.86(m,1H),8.55–8.52(m,2H),7.87–7.85(m,1H),7.76–7.73(m,1H ),7.40(s,1H),6.51(s,1H), 4.75-4.74(m,2H),4.22-4.19(t,2H),3.58-3.52(m,108H),2.82(t,2H), 2.50-2.47 (m, 2H), 2.04-1.98 (m, 2H), 0.94–0.90 (m, 3H).
Embodiment 2
[0031] Preparation of 4arm-PEG124-DOX(III)
[0032] 10 mmol equivalent of 4-arm-PEGm-Hz-I and 8 mmol of doxorubicin hydrochloride were dissolved in anhydrous DMSO solvent, and 5 mmol% catalytic equivalent of phosphoric acid was added to the organic solvent. React at a reaction temperature of 25° C. in the dark for 54 hours, check that the reaction is complete, and recrystallize through column chromatography to obtain the target product. Yield: 81.2%. The NMR data are as follows: 1 H NMR (400MHz,DMSO)δ,9.05(s,1H),8.89–8.86(m,1H), 8.55–8.52(m,2H),7.87–7.85(m,1H),7.76–7.73(m,1H ),7.40(s,1H),6.51(s,1H),4.75-4.74(m,2H),4.22-4.19(t,2H),3.58-3.52(m,508H),2.82(t,2H), 2.50-2.47(m,2H),2.04-1.98(m,2H),0.94–0.90(m,3H).
Embodiment 3
[0034] Preparation of 4arm-PEG240-DOX(III)
[0035] 10 mmol equivalent of 4-arm-PEG240-Hz-I and 10 mmol of doxorubicin hydrochloride were dissolved in anhydrous chloroform solvent, and 10 mmol% catalytic equivalent of phosphoric acid was added to the organic solvent. React at a reaction temperature of 30° C. in the dark for 60 hours, check that the reaction is complete, and recrystallize through column chromatography to obtain the target product. Yield: 85.4%. The NMR data are as follows: 1 H NMR (400MHz,DMSO)δ,9.05(s,1H),8.89–8.86(m,1H), 8.55–8.52(m,2H),7.87–7.85(m,1H),7.76–7.73(m,1H ),7.40(s,1H),6.51(s,1H),4.75-4.74(m,2H),4.22-4.19(t,2H),3.58-3.52(m,972H),2.82(t,2H), 2.50-2.47(m,2H), 2.04-1.98(m,2H),0.94–0.90(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com