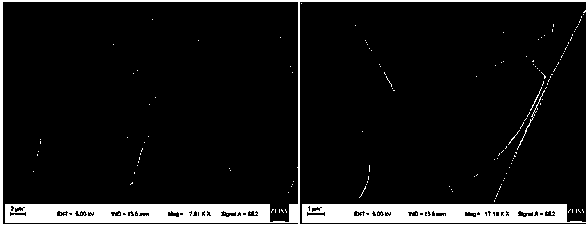
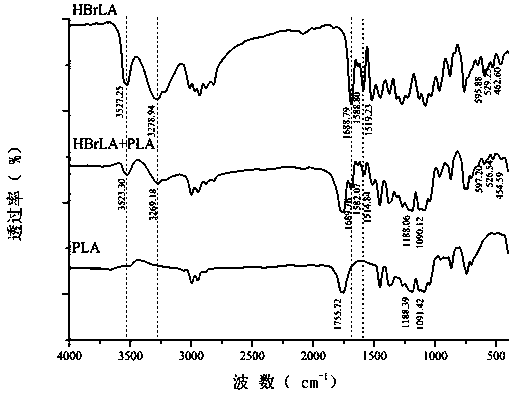
Polylactic acid/lappaconite hydrobromide composite fiber and preparation and application thereof
A technology of homogenin hydrobromide and composite fiber, which is applied in medical science, bandages, absorbent pads, etc., can solve the problem of single performance, and achieve good biocompatibility and good drug controllable release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Preparation of spinning solution: use an electronic balance to accurately weigh 2.95g of polylactic acid, add it to 20ml of dichloromethane, and continue stirring for 6-12 hours until the polymer solution is evenly mixed and transparent; use an electronic balance to accurately weigh 20mg of hydrobromic acid Ujiasu was added to the mixed solution, stirred for 3 hours at a speed of 200r / min, and then ultrasonically removed for 1h at a power of 50kHz to obtain a uniform and transparent spinning solution;
[0038] Electrospinning: Fill the spinning solution into a 10ml syringe, then connect the positive pole of the high voltage power supply to the syringe needle tip of the syringe pump, and select the needle with an outer diameter of 0.6 mm and a length of 50 mm to connect the negative pole of the high voltage power supply to aluminum foil. Under the conditions of controlling the spinning temperature at 20~30°C and the humidity at 20~50%, adjust the electrospinning parameters...
Embodiment 2
[0042] Preparation of spinning solution: Accurately weigh 1.69g of polylactic acid with an electronic balance, add it to 20ml of dichloromethane, and continue stirring for 6-12 hours until the solution is evenly mixed and transparent; accurately weigh 10mg of polylactic acid, and Add it into the mixed solution and stir for 3 hours at a speed of 200r / min to obtain a uniform and transparent spinning solution;
[0043] Electrospinning: Put the spinning solution into a 10ml syringe, then connect the positive pole of the high-voltage power supply to the syringe needle tip of the syringe pump, select the needle specification as 0.6mm in outer diameter and 1.5mm in length, and connect the negative pole of the high-voltage power supply to aluminum foil; control Under the conditions of spinning temperature of 20~30℃ and humidity of 20~50%, adjust the electrospinning parameters: the distance between the nozzle and the receiving drum is 10cm, the spinning voltage is 5.1kV, and the nozzle ...
Embodiment 3
[0047] Preparation of spinning solution: Accurately weigh 2.31g of polylactic acid, add it to 20ml of dichloromethane, and keep stirring for 6-12 hours until the solution is evenly mixed and transparent; solution, stirred at a rotating speed of 200r / min for 3 hours to obtain a uniform and transparent spinning solution;
[0048] Electrospinning: Fill the spinning liquid into a 10ml syringe, then connect the positive pole of the high-voltage power supply to the syringe needle tip of the syringe pump. The needle specifications are selected to be 0.6 mm in outer diameter and 1.5 mm in length, and the negative pole of the high-voltage power supply is connected to aluminum foil. Under the conditions of controlling the spinning temperature at 20~30°C and the humidity at 20~50%, adjust the electrospinning parameters: the distance between the nozzle and the receiving drum is 11cm, the spinning voltage is 7.07kV, and the nozzle speed is 1ml / min , the nozzle speed is 1ml / min, the drum sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com