Detection method of ranavirus in water
A detection method, frog virus technology, applied in the directions of microorganism-based methods, biochemical equipment and methods, and microbial determination/inspection, etc., to avoid false negatives and to be easily identified.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Water sample virus enrichment:
[0061] Take 1L of aquaculture water sample and use medical gauze to filter out impurities, and use 5mol / L HCl to adjust the pH of the filtered water sample to 3.5. Add 10gNaCl and 24gAlCl to the adjusted pH water sample 3 ·6H 2 O to stand for 5 hours. After standing still, a flocculent precipitate will be formed at the bottom. Pour off part of the supernatant. Use a 0.45 μL filter membrane to filter out the excess water at the bottom of the flocculent precipitate. Dry the filtered flocculent precipitate and put it into an EP tube. , add 1mL of PBS buffer solution and pipette to dissolve, and the obtained solution is the solution after the water body virus is enriched.
[0062] DNA extraction:
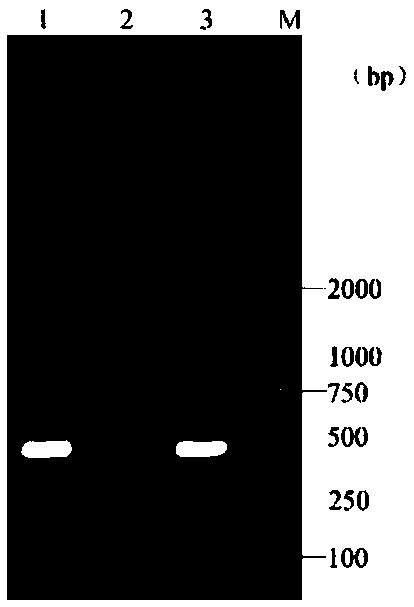
[0063] After the virus-enriched solution was repeatedly frozen and thawed three times, 1 mL was drawn into a test tube, and centrifuged at 13,000 r / min for 5 min at a temperature of 4°C. Take 400 μL of centrifuged supernatant, add 500 μL of sa...
Embodiment 2
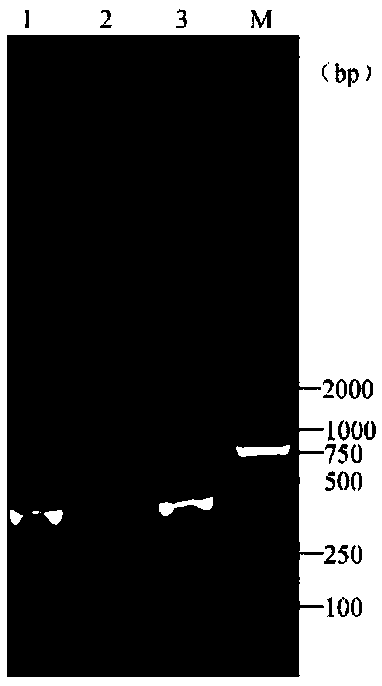
[0077] Take 1L of tap water, add 2mL of TCID to the tap water 50 The frog virus cell culture fluid of 10.648 is used as the aquaculture water sample to be tested, and the steps in Example 1 are used to successively carry out water sample virus enrichment, DNA extraction and PCR detection, and finally use the product after the PCR reaction after agarose gel electrophoresis The gel imager takes pictures such as figure 2 As shown, a main band appears between maker 250bp-500bp in lane 1, which is judged to be positive for RAV, indicating that frog virus was detected. Then take 1L of tap water without adding any reagents and follow the steps in Example 1 to carry out water sample virus enrichment, DNA extraction and PCR detection in sequence, and finally the product after the PCR reaction is subjected to agarose gel electrophoresis and then photographed with a gel imager as shown figure 2 No bands were added in the 2 swimming lanes shown, and it was judged to be RAV negative, wh...
Embodiment 3
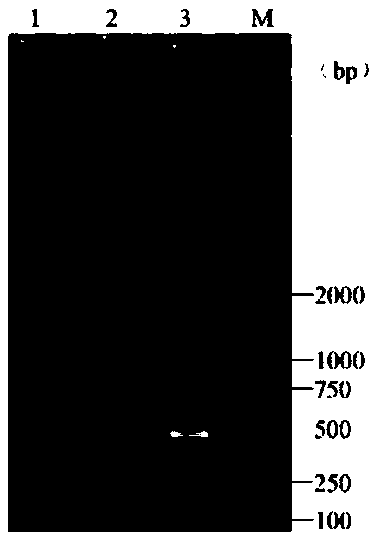
[0079] Take 1L of well water and add 2mL of TCID to the well water 50 The frog virus cell culture fluid of 10.648 is used as the aquaculture water sample to be tested, and the steps in Example 1 are used to successively carry out water sample virus enrichment, DNA extraction and PCR detection, and finally use the product after the PCR reaction after agarose gel electrophoresis The gel imager takes pictures such as image 3 A main band appears between maker250bp-500bp in lane 1 shown, which is judged to be positive for RAV, indicating that frog virus was detected. Then take 1L of well water without adding any reagents and follow the steps in Example 1 to carry out water sample virus enrichment, DNA extraction and PCR detection in sequence, and finally perform agarose gel electrophoresis on the products after the PCR reaction, and use a gel imager to take pictures as follows: image 3 No bands were added in the 2 lanes shown, and it was judged to be negative for RAV, indicating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com