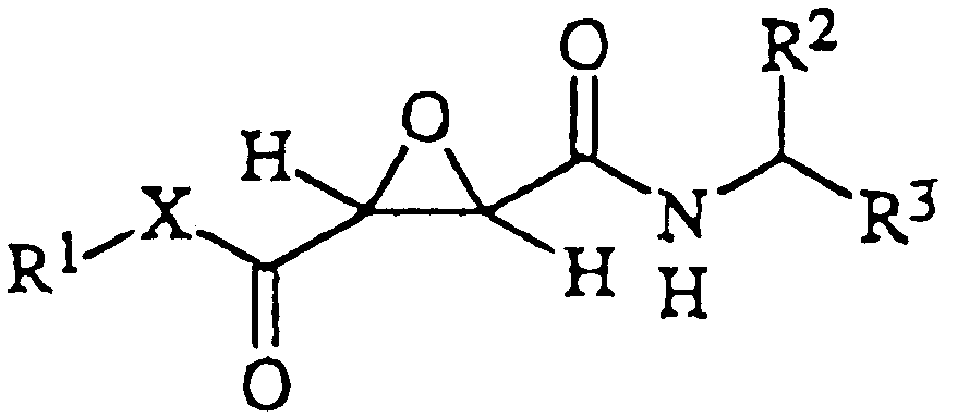
Epoxysuccinic acid derivatives
A technology of epoxy succinic acid and derivatives, which can be used in pharmaceutical combinations, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as not yet disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 (2S, 3S)-3-diphenylmethylcarbamoyloxirane-2-carboxylic acid ethyl ester (No. 2 compound)
[0063] To a solution of (2S, 3S)-3-ethoxyformyloxirane-2-carboxylic acid (8.01g, 50.0mmol) in ethyl acetate (120ml), N-hydroxysuccinate was added successively under ice cooling Imide (5.76 g, 50.0 mmol) and N,N'-dicyclohexylcarbodiimide (5.75 g, 50.0 mmol). The mixture was stirred at 5°C for 1 hour, and a solution of aminodiphenylmethane (9.17 g, 50.0 mmol) in ethyl acetate (30 ml) was added. The mixture was stirred at 5°C for 1 hour and at room temperature for another 1 hour. The resulting N,N'-dicyclohexylurea (DC-Urea) was filtered off, and the filtrate was washed successively with 1N hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated aqueous sodium chloride, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the residue was recrystallized from ethyl acetate-n-hexane to obtain the desired white crys...
Embodiment 2
[0067] Example 2 (2S, 3S)-3-diphenylmethylcarbamoyloxirane-2-carboxylic acid (No. 1 compound)
[0068] Ethyl (2S,3S)-3-diphenylmethylcarbamoyloxirane-2-carboxylate (5.50 g, 16.9 mmol) from Example 1 was dissolved in ethanol (300 ml). To the solution was added 0.5N sodium hydroxide ethanol solution (40.4ml, 20.2mmol), and the mixture was stirred at room temperature for 3 hours. The solvent was evaporated under reduced pressure, water (300ml) was added and washed with two 100ml portions of ether. The pH of the aqueous phase was adjusted to 1-2 with 2N hydrochloric acid, then extracted with three 100ml portions of ethyl acetate. The organic phase was washed with saturated aqueous sodium chloride and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain the desired white powdery crystalline product (4.76 g, yield: 95%). 1 H-NMR (400MHz, CD 3 OD) δ:
[0069] 3.57(1H,d,J=2Hz), 3.71(1H,d,J=2Hz), 6.23
[0070] (1H, s), 7.2-7.4 (10H, m...
Embodiment 3
[0071] Example 3 (2S, 3S)-3-diphenylmethylcarbamoyloxirane-2-sodium carboxylate (sodium salt of No. 1 compound)
[0072] The compound obtained in Example 2 (100 mg, 0.336 mol) was dissolved in ethyl acetate (10 ml). To the resulting solution was added a solution of sodium bicarbonate (28.2mg, 0.336mmol) in water (10ml). The mixture was shaken vigorously. The aqueous phase was separated, then concentrated to dryness under reduced pressure to obtain the desired product as a white powder (106 mg, yield: 99%). 1 H-NMR (400MHz, D 2 O) δ:
[0073] 3.47(1H,d,J=2Hz), 3.63(1H,d,J=2Hz), 6.15
[0074] (1H, s), 7.3-7.5(10H, m).IR(KBr)cm -1 :
[0075] 3412, 3219, 3064, 3030, 1670, 1632, 1552, 1495,
[0076] 1454, 1448, 1398, 1317, 1285, 1248, 1093, 1086,
[0077] 1051, 1030, 1007, 960, 899, 862, 783, 758, 742,
[0078] 698, 677, 642, 602, 571, 482.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com