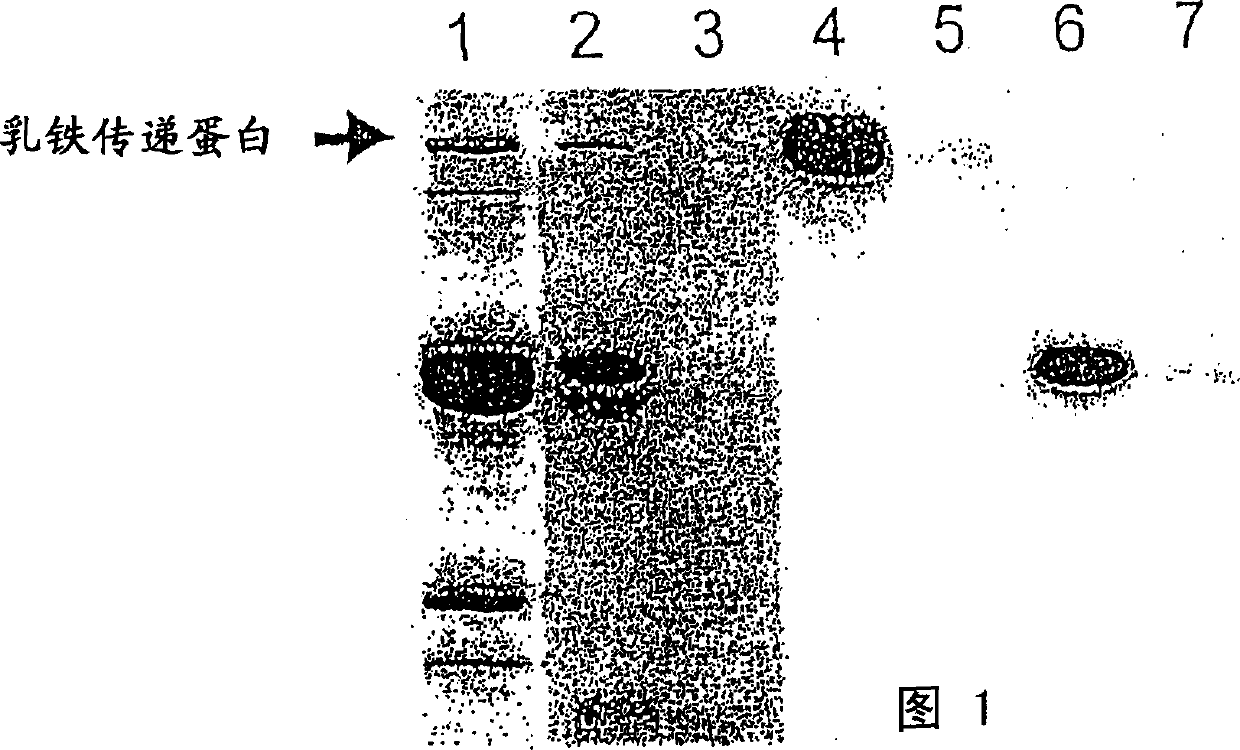
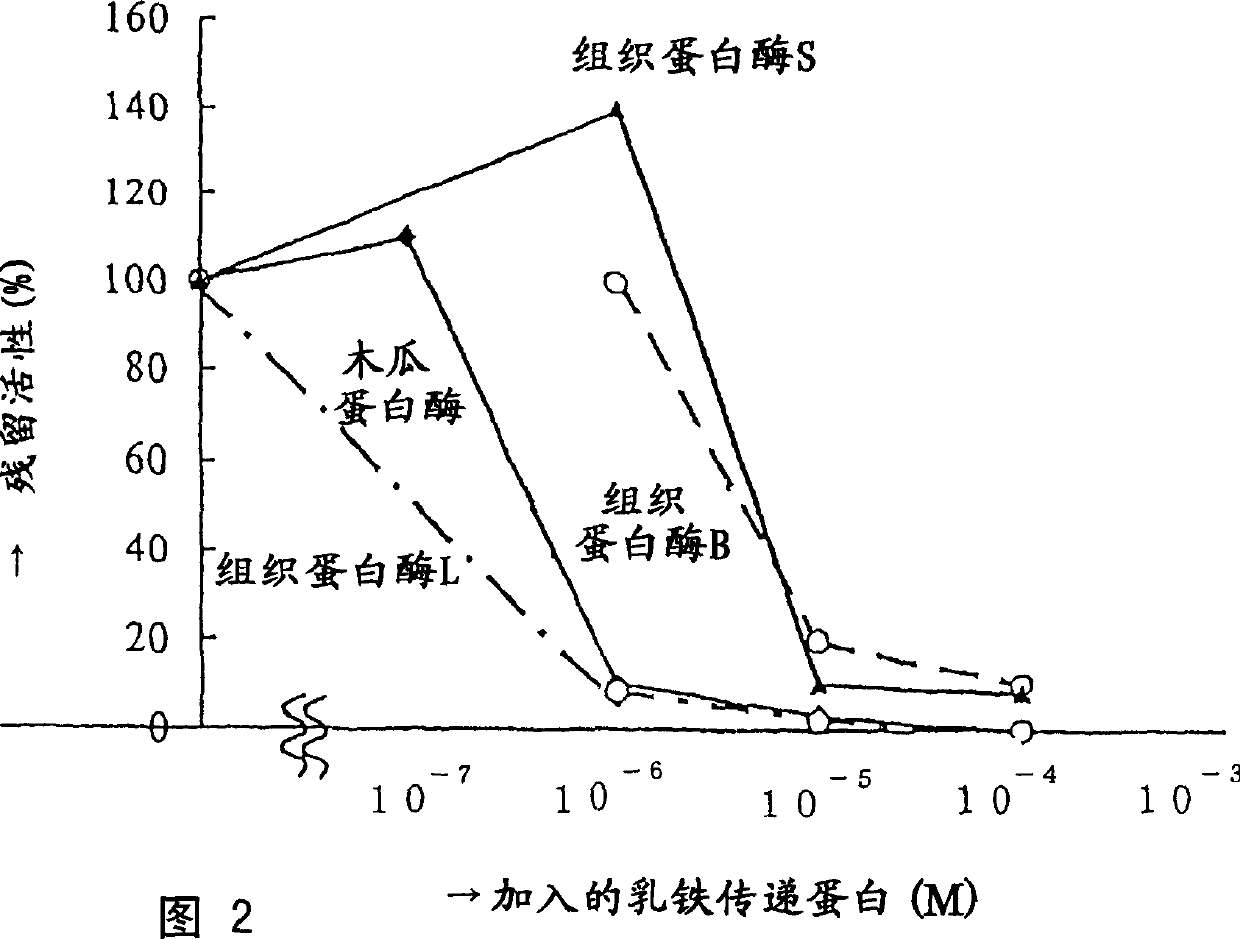
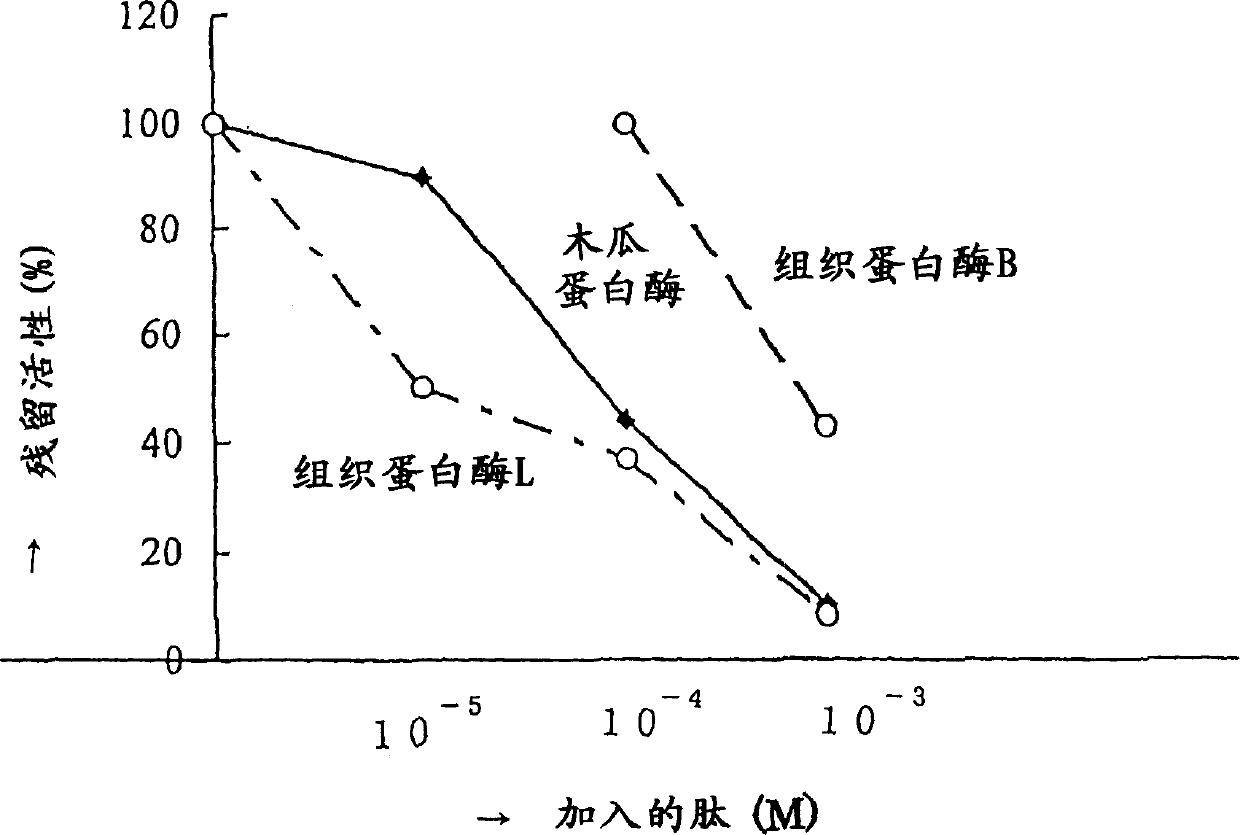
Protease inhibitor
An inhibitor, cysteine protease technology, applied in the direction of protease inhibitors, transferrin, peptide/protein components, etc., can solve the problem of unknown lactoferrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] The peptide having the amino acid sequence 679-695 of SEQ ID No. 1 in the sequence table was produced according to the following steps (Fig. 3, human lactoferrin peptide Y679-K695).
[0157] The above peptide of the present invention was produced by synthesis using an automated peptide synthesizer (manufactured by Applied Biosystems, Model 433A).
[0158] With N-methylpyrrolidone (product of Applied Biosystems, hereinafter abbreviated as NMP) containing 20% piperidine, the amino protecting group Fmoc- The group was cleaved and the resin was washed with NMP. Then, using the FastMoc (registered trademark) kit (product of Applied Biosystems) to make Fmoc-threonine [precisely, Fmoc-amino acid (product of Applied Biosystems) )] was condensed on the resin, and the resin was washed with NMP. The Fmoc group is then cleaved and Fmoc-alanine corresponding to the second amino acid at the C-terminal is condensed, followed by washing the resin. By further repeating the steps of...
Embodiment 2
[0162] (preparation of tablets formulated with lactoferrin)
[0163] Cystatin tablets containing the following compositions were produced as follows.
[0164] Bovine lactoferrin (product of Morinaga dairy company) 40.0%
[0165] Lactose (Morinaga Dairy Company product) 18.5%
[0166] Corn starch (Nisshin flour milling company product) 30.7%
[0167] Magnesium stearate (product of Taihei Chemical Industry Co., Ltd.) 1.4%
[0168] Carboxymethylcellulose calcium (Gotokn chemical company product) 9.4%
[0169] Add an appropriate amount of sterile purified water to knead the mixture of bovine lactoferrin, lactose, cornstarch and carboxymethylcellulose calcium), and dry the kneaded material at 50° C. for 3 hours. Magnesium stearate was then added to the obtained dry product and mixed uniformly, followed by tableting according to conventional procedures, thereby obtaining tablets.
Embodiment 3
[0171] (preparation of lactoferrin capsules)
[0172] With a 50-mesh sieve (product of Yamato Science Co., Ltd.), 600 g of lactose (product of Wako Pure Chemical Industry Co., Ltd.), 400 g of cornstarch (product of Nissnin Powder Milling Co., Ltd.), 400 g of crystalline cellulose (product of Wako Pure Chemical Industry Co., Ltd.) and 600 g One gram of bovine lactoferrin (product of Morinaga Dairy Company) was sieved, packed into a polyethylene bag with a thickness of 0.5 mm, and mixed up and down repeatedly. Using a fully automated capsule filling machine (manufactured by Cesere Pedini, press-fit type), the obtained powder is filled into capsules (Shionogi Qualicaps company product, No. 1 gelatin capsule, OP yellow No. 6 shell, empty weight 75 mg) with a capacity of 275 mg , thus obtaining 7000 capsules, each containing 82 mg of bovine lactoferrin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com