Protease inhibitors
A compound and medicinal salt technology, applied in the field of protease inhibitors, can solve problems such as poor solubility, lack of selectivity, and too fast serum clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0211] Example number Chemical name
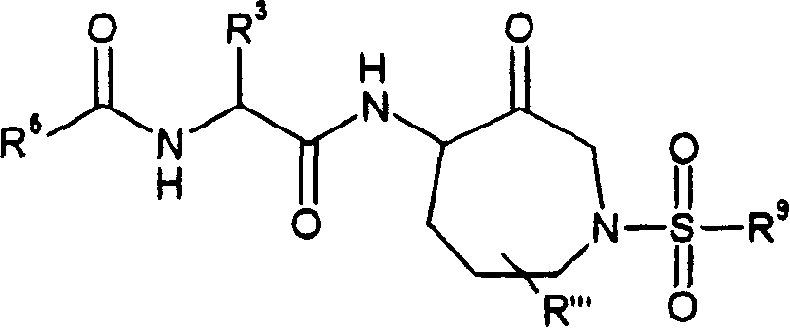
[0212] 1 {(S)-1-[1-((S)-2-benzyloxycarbonylamino-4-methyl-pentanoyl
[0213] Base)-3-oxo-azepan (azepan)-4-ylcarbamoyl}
[0214] Benzyl carbamate
[0215] 2 Naphthylene-2-carboxylic acid [(S)-1-(1-benzyl-3-oxo-azepane-
[0216] 4-ylcarbamoyl)-3-methyl-butyl]amide
[0217] 3 Benzo[1,3]dioxol-5-carboxylic acid [(S)-1-(1-benzyl-
[0218] 3-oxo-azepan-4-ylcarbamoyl)-3-methyl-butyl]
[0219] Amide
[0220] 4 Benzofuran-2-carboxylic acid [(S)-1-(1-benzyl-3-oxo-azepane
[0221] Alk-4-ylcarbamoyl)-3-methyl-butyl]amide
[0222] 5 Benzo[b]thiophene-2-carboxylic acid[(S)-1-(1-benzyl-3-oxo-azacycle
[0223] Heptane-4-ylcarbamoyl)-3-methyl-butyl]amide
[0224] 6 naphthylene-2-sulfonyl[(S)-1-(1-benzyl-3-oxo-azepanyl
[0225] Alk-4-ylcarbamoyl)-3-methyl-butyl]-amide
[0226] 7 Quinoline-2-carboxylic acid [(S)-1-(1-benzyl-3-oxo-azepane-4-
[0227] Carbamoyl)-3-met...
Embodiment 192
[0977] The deuterated compound of Example 192 can be conveniently prepared according to Scheme 4. Those skilled in the art will understand from Example 192 and Scheme 4 how to prepare any of the deuterated compounds of the invention.
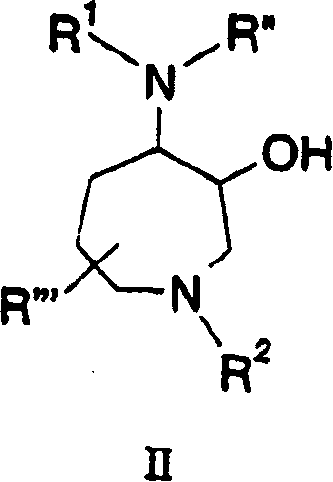
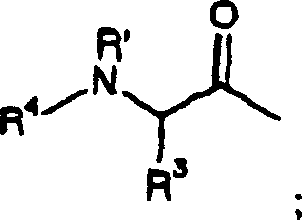
[0978] Single diastereoisomer benzofuran-2-carboxylic acid {(S)-3-methyl-1-[(2,2′,4-trideutero)-3-oxo-1-(pyridine- 2-Sulfonyl)-azepan-4-ylcarbamoyl]-butyl} amides 31 and 32 can be prepared as shown in Scheme 4. Alkylation of benzyl allylcarbamate 22 with 5-bromo-1-pentene in the presence of a base such as sodium hydride affords diene 23. Treatment of diene 23 with bis(tricyclohexylphosphine)phenylmethyleneruthenium(IV) dichloride by the method of Grubbs afforded 2,3,4,7-tetrahydro-azepine-1-carboxylic acid Benzyl esters 24. Epoxidation of azepane 24 with standard oxidizing agents common in the art, such as m-CPBA, affords epoxide 25. The nucleophilic epoxy ring of 25 can be opened with a reagent such as sodium azide to give the azido alcohol...
Embodiment 1
[1080] Preparation of {(S)-1-[1-((S)-2-benzyloxycarbonylamino-4-methyl-pentanoyl)-3-oxo-azepan-4-ylcarbamoyl ]} benzyl carbamate
[1081] a.) Allyl-pent-4-enyl-tert-butyl carbamate
[1082] To a suspension of NaH (3.05 g, 76.33 mmol of 60% NaH in oil; washed with hexanes) in DMF (30 mL) was added tert-butyl N-allylcarbamate (6.0 g, 38.2 mmol) dropwise . The mixture was stirred at room temperature for about 10 minutes, and 5-bromo-1-pentene (6.78 mL, 57.24 mmol) was added dropwise. The reaction was heated to 40°C for about 2 hours, and the reaction was partitioned between ethyl acetate and water. The organic layer was washed with water (2x), brine, dried (magnesium sulfate), filtered and concentrated to afford 10 g of the title compound as an oil: MS (EI) 226 (M+H + ).
[1083] b.) tert-butyl 2,3,4,7-tetrahydro-azepane-1-carboxylate
[1084] To a solution of Example 1a (4.5 g) in benzene was added molybdenum 2,6-diisopropylphenyliminomethyl-2-phenylpropyl bis-tert-butoxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com