Method of analyzing and determining genotoxic impurities in valsartan by HPLC
A genotoxicity and impurity technology, applied in analytical materials, measuring devices, material separation, etc., can solve problems such as genotoxic impurities of valsartan not seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
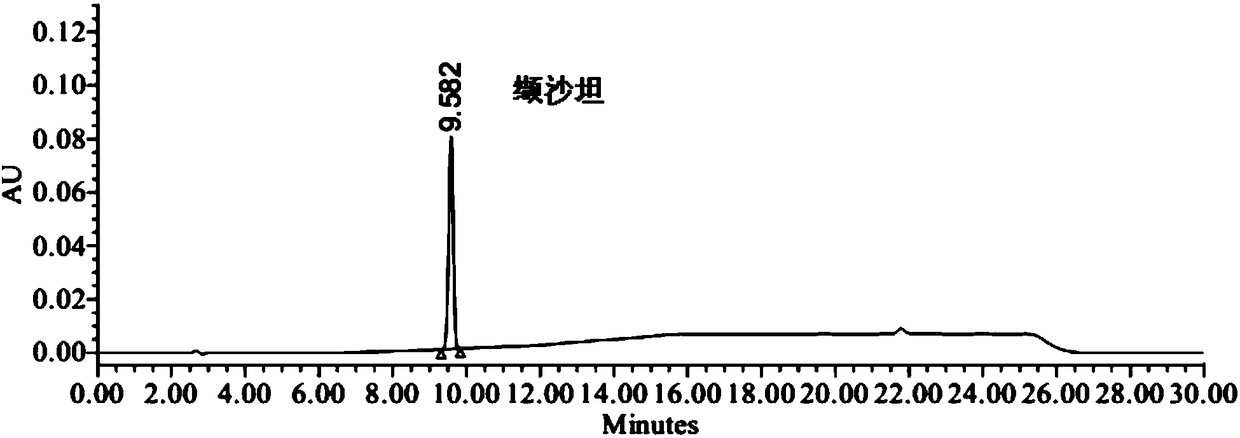
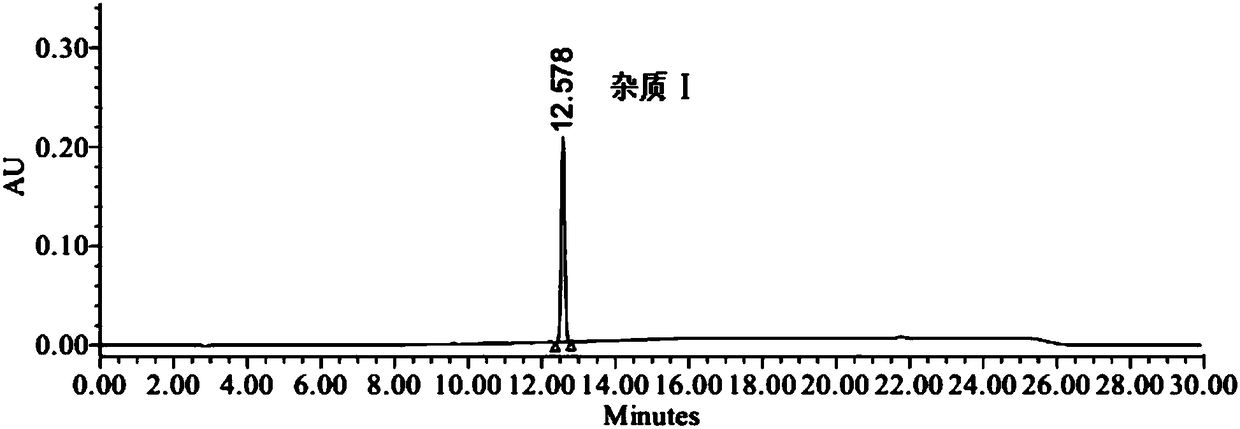
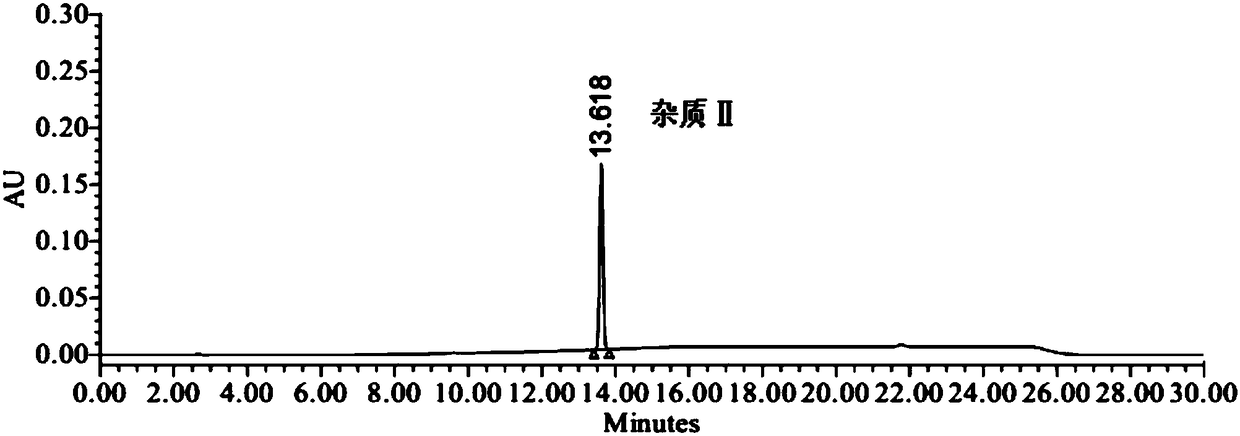
[0035] Embodiment 1 specificity and impurity localization test
[0036] (1) Preparation of sample solution
[0037] Solvent: 90% acetonitrile in water
[0038] Positioning solution: Take the appropriate amount of valsartan, impurity Ⅰ and impurity Ⅱ, weigh them accurately, place them in different measuring bottles, add solvent (90% acetonitrile aqueous solution) to dissolve and dilute to make valsartan, impurity Ⅰ, impurity Ⅱ II. Each 0.1 mg / ml solution is used as a compound localization solution.
[0039] Specific solution: take 1.0 mg of impurity Ⅰ and impurity Ⅱ, weigh them accurately, put them in the same 1000ml measuring bottle, dissolve and dilute to 1000ml with solvent (90% acetonitrile aqueous solution), shake well, and store them as impurity Ⅰ and impurity Ⅱ Take 200mg of valsartan, accurately weighed, put it in a 10ml measuring bottle, and simultaneously accurately measure 1ml of impurity I and impurity II stock solution and put it in the measuring bottle, add solv...
Embodiment 2
[0054] Embodiment 2 limit of quantification
[0055] (1) Preparation of sample solution
[0056] Measurement of baseline noise: Precisely measure 20 μl of solvent, inject it into a liquid chromatograph, inject 3 needles continuously, and detect according to the chromatographic conditions described in the embodiment of the present invention. Record the noise of the blank baseline within the peak time range of impurity Ⅰ and impurity Ⅱ, and calculate the average value.
[0057] Preparation of quantitation limit solution: take appropriate amount of impurity I and impurity II, weigh them accurately, put them in the same measuring bottle, dissolve them with a solvent and gradually dilute them, and detect them according to the chromatographic conditions described in the examples of the present invention until they are diluted to impurity I and impurity II. The ratio of the peak height of II to the corresponding noise (i.e. signal-to-noise ratio, S / N) is about 10. At this time, the ...
Embodiment 3
[0064] Example 3 Linearity
[0065] (1) Sample solution preparation
[0066] Accurately weigh 20 mg each of impurity Ⅰ and impurity Ⅱ, put them in the same 100ml measuring bottle, add solvent to dissolve and dilute to the mark, and shake well; accurately measure 1ml, put it in a 10ml measuring bottle, add solvent to dilute to the mark, shake well, and use as Linear stock solution.
[0067] Precisely measure 1ml, 2ml, 3ml, 5ml, 7.5ml, 10ml, 15ml of the linear stock solution, put them in different 100ml measuring bottles, add solvent to dilute to the mark, shake well, and use them as 20%, 40%, and 60% of the limit respectively. %, 100%, 150%, 200%, 300% linear solution. According to the chromatographic conditions described in the present invention, the linear equation and the correlation coefficient are calculated.
[0068] (2) Chromatographic conditions
[0069] Chromatographic condition is the same as embodiment 1
[0070] (3) Test results
[0071] The test results show ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com