Colorimetric detection method for nitrite in water
A technology of nitrite and detection method, applied in the field of analytical chemistry, can solve problems such as secondary pollution of the environment, and achieve the effects of simple operation, simple and cheap instruments and equipment, and simplified detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
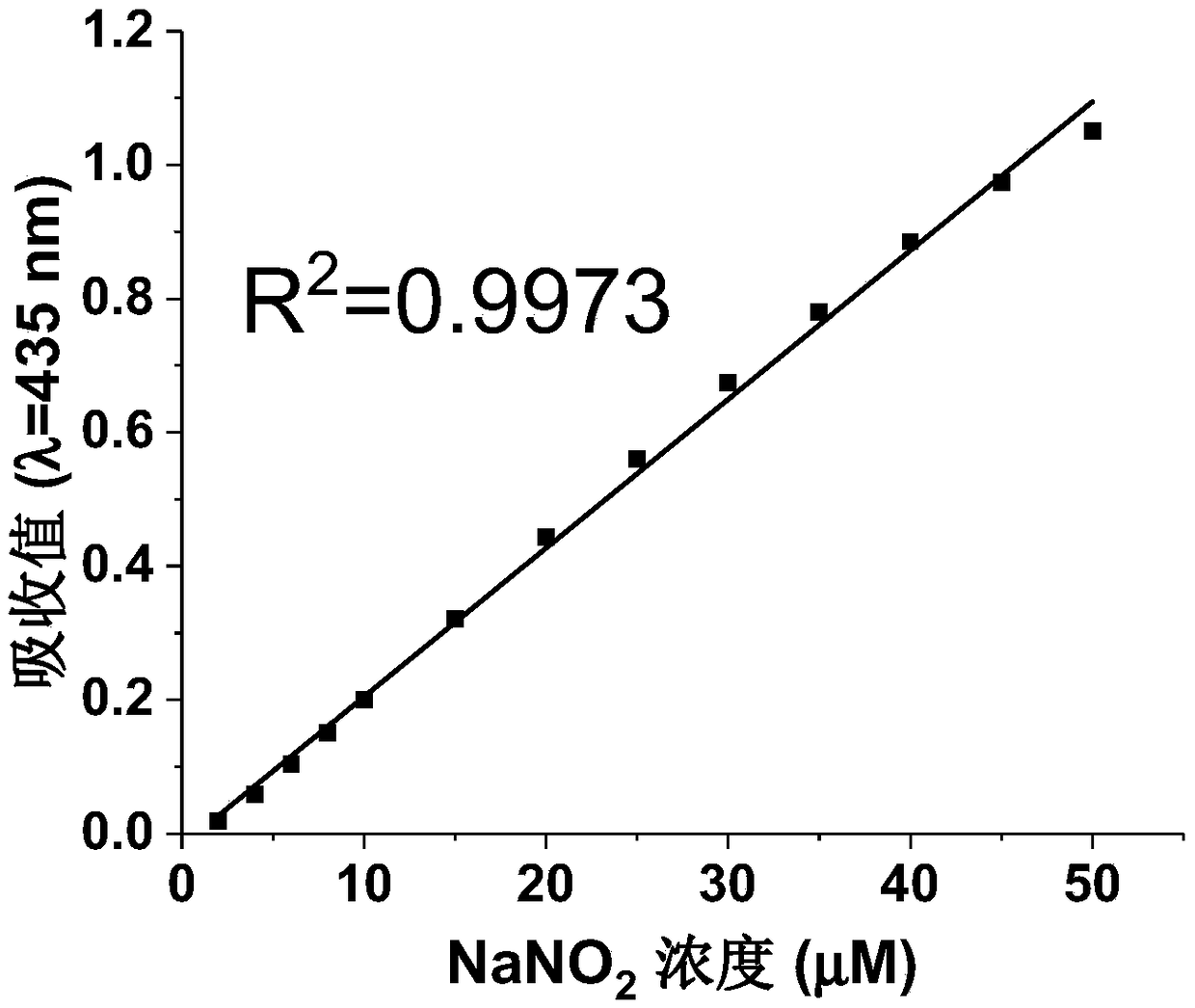
[0030] Embodiment 1: Utilize the aqueous solution of 2mM 2-amino-6-chlorobenzoic acid to detect the concentration of nitrite ion
[0031] The selected reagent is 2-amino-6-chlorobenzoic acid, that is, when the R substituent in the compound of general formula I is chlorine, the molecular weight is 171.
[0032] Preparation of 2-amino-6-chlorobenzoic acid aqueous solution: Weigh 171mg of 2-amino-6-chlorobenzoic acid, dissolve it in double distilled water or deionized water, and make the volume to 500mL, with a final concentration of 2mM.
[0033] Prepare NaNO 2 Standard solution: Weigh 172.5 mg of sodium nitrite (molecular weight: 69), dissolve it in double distilled water or deionized water, and then dilute it to 500 mL with double distilled water or deionized water step by step to 13 different concentration gradients of NaNO2 The standard solutions are 2, 4, 6, 8, 10, 15, 20, 25, 30, 35, 40, 45 and 50 (in mM), each standard solution is 50mL, and the final concentration concen...
Embodiment 2
[0036] Embodiment 2: Utilize the aqueous solution of 0.5mM 2-amino-6-chlorobenzoic acid to detect the concentration of nitrite ion
[0037] The selected reagent is 2-amino-6-chlorobenzoic acid, that is, when the R substituent in the compound of general formula I is chlorine, the molecular weight is 171.
[0038] Preparation of 2-amino-6-chlorobenzoic acid aqueous solution: Weigh 43mg of 2-amino-6-chlorobenzoic acid, dissolve it in double distilled water or deionized water, and adjust the volume to 500mL with a final concentration of 0.5mM.
[0039] Prepare NaNO 2 Standard solution: Weigh 172.5 mg of sodium nitrite (molecular weight: 69), dissolve it in double distilled water or deionized water, and then dilute to 500 mL with double distilled water or deionized water step by step to 12 different concentration gradients of NaNO2 The standard solutions are 0, 1, 2, 3, 4, 6, 8, 10, 15, 20, 25 and 30 (in mM), each standard solution is 50mL, and the final concentration gradient can...
Embodiment 3
[0042] Embodiment 3: Utilize the aqueous acetic acid solution of 2mM 2-amino-6-chlorobenzoic acid to detect the concentration of nitrite ion
[0043] The selected reagent is 2-amino-6-chlorobenzoic acid, that is, when the R substituent in the compound of general formula I is chlorine, the molecular weight is 171.
[0044] For the preparation of 2-amino-6-chlorobenzoic acid acetic acid aqueous solution, the concentration of 2-amino-6-chlorobenzoic acid is generally controlled at 0-30mM, the concentration of acetic acid is generally 0.1-1M, and the pH value is 3.0-4.0. Accurately measure 40mL of glacial acetic acid, dilute to 1000mL with double distilled water or deionized water, the final concentration of acetic acid aqueous solution is 0.7M; weigh 171mg of 2-amino-6-chlorobenzoic acid, dissolve it with 0.7M Make up to 500mL to obtain 2-amino-6-chlorobenzoic acid acetic acid aqueous solution.
[0045] Prepare NaNO 2 Standard solution: Weigh 172.5 mg of sodium nitrite (molecul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com