Amphiphilic polypeptide P13 and preparation method thereof
An amphiphilic, P13 technology, applied in the field of peptides, can solve problems such as reducing drug utilization, achieve high drug utilization, and improve the effect of acid-base buffering capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
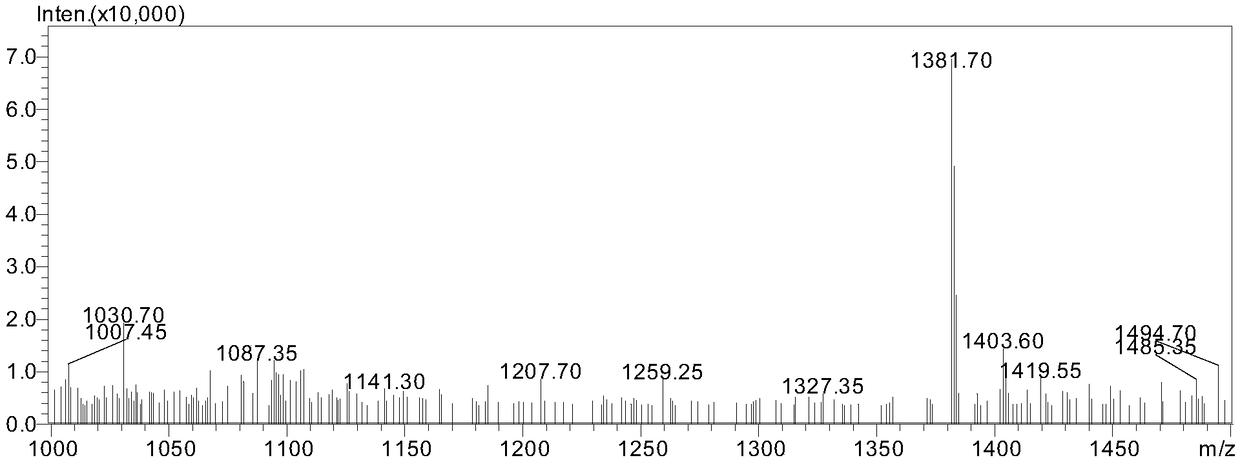
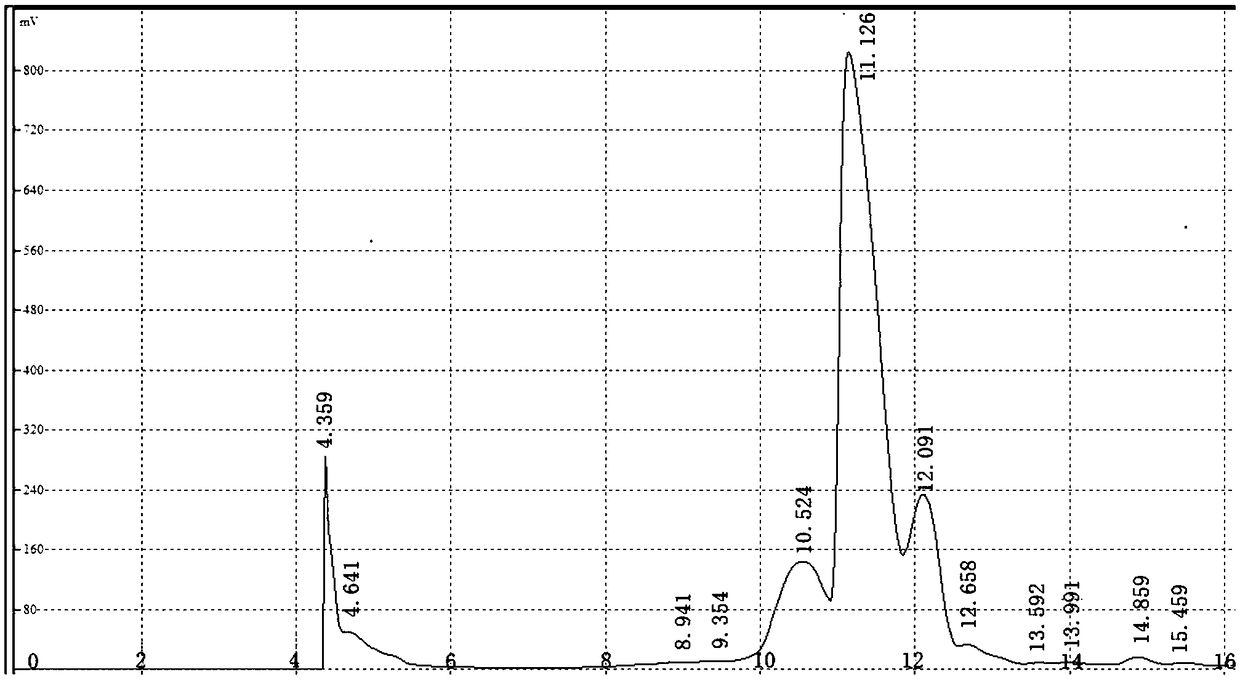
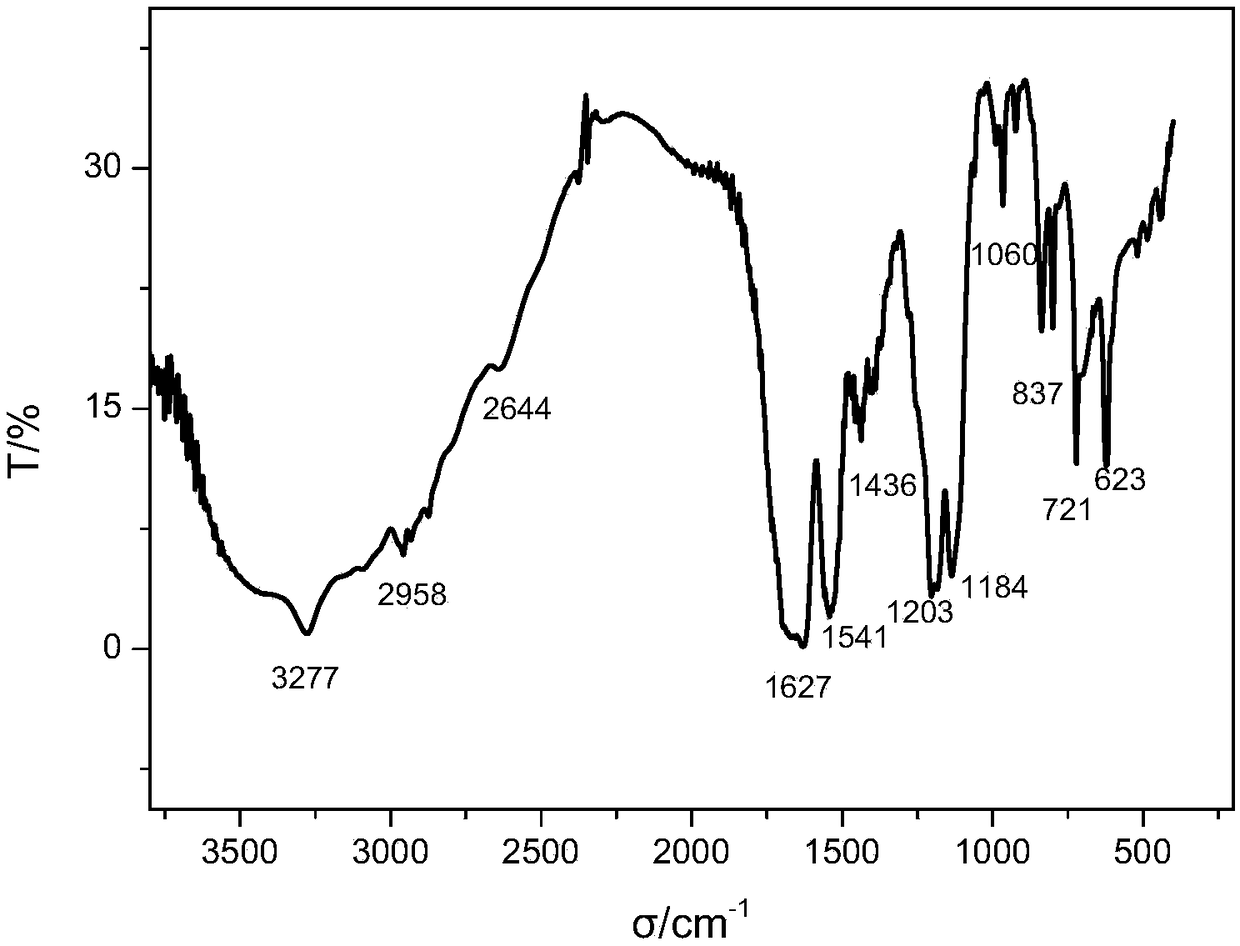
Image
Examples
preparation example Construction
[0054] The present invention also provides a method for preparing the above-mentioned amphiphilic polypeptide P13, comprising:
[0055] 1) Soak the matrix resin in the solvent, then add N, N-diisopropylethylamine (DIEA), aspartic acid Fmoc-Asp (OtBu)-OH to carry out contact reaction, then wash, head, remove protection, and finally washing until ninhydrin is detected as blue to obtain a secondary resin;
[0056] 2) The secondary resin is subjected to multiple modification treatments with various compounds in turn to obtain a tertiary resin; each modification process is as follows: the secondary resin and the compound are mixed in 1-hydroxybenzotriazole (HoBt), benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate (HBTU) in the presence of contact reaction, and then through deprotection, washing until ninhydrin is detected as Blue to obtain a tertiary resin; the compounds in a single modification process are independently Fmoc-Gly-OH, Fmoc-Arg(pbf)-OH, Fmoc-His(OtBu...
Embodiment 1
[0076] 1) Soak 0.25g of 2-chlorotrityl chloride resin in 5mL, 15°C DCM for 10min, then add 0.3mLDIEA, 0.08gFmoc-Asp(OtBu)-OH, react at 15°C for 30min, and then wash;
[0077] 2) Add 5mL DCM, 0.3mL methanol and 0.3mL DIEA to the reaction system for capping treatment, then add piperidine for deprotection, and then wash the reaction system until ninhydrin is detected as blue;
[0078] 3) Add 0.2g Fmoc-Gly-OH, 0.2g HoBt, and 0.2g HBTU to the reaction system and contact with it for 30 minutes at 15°C. After washing, add piperidine for deprotection, and wash the reaction system again until it passes ninhydrin detection It is blue; sequentially add 0.5g Fmoc-Arg(pbf)-OH, 0.5g Fmoc-His(OtBu)-OH, 0.2g Fmoc-Gly-OH, 0.2g Fmoc-Ala-OH and react at 15°C for 30min;
[0079] 4) Wash with DMF, DCM and methanol in sequence, then dry at 30°C for 1 h to powder, add 10 mL of cutting solution A (the volume ratio of TFA, TIS, EDT and water is 95:1.5:1.5:2) to cut the resin and Peptide chain, add ic...
Embodiment 2
[0082] 1) Soak 0.25g of 2-chlorotrityl chloride resin in 5mL, 20°C DCM for 20min, then add 0.5mLDIEA, 0.2gFmoc-Asp(OtBu)-OH, react at 20°C for 60min, and then wash;
[0083] 2) Add 10mL DCM, 0.5mL methanol and 0.5mL DIEA to the reaction system for capping treatment, then add piperidine for deprotection, and then wash the reaction system until ninhydrin is detected as blue;
[0084] 3) Add 0.4g Fmoc-Gly-OH, 0.3g HoBt, and 0.3g HBTU into the reaction system and contact with the reaction for 60min at 20°C. After washing, add piperidine for deprotection, and wash the reaction system again until it is detected by ninhydrin It is blue; sequentially add 0.6g Fmoc-Arg(pbf)-OH, 0.6g Fmoc-His(OtBu)-OH, 0.4g Fmoc-Gly-OH, 0.4g Fmoc-Ala-OH and react at 20°C for 60min;
[0085] 4) Wash with DMF, DCM, and methanol in sequence, then dry at 30°C for 1 h to powder, add 12 mL of cutting solution A (the volume ratio of TFA, TIS, EDT, and water is 95:2:2:1) to cut the resin and Peptide chain, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com