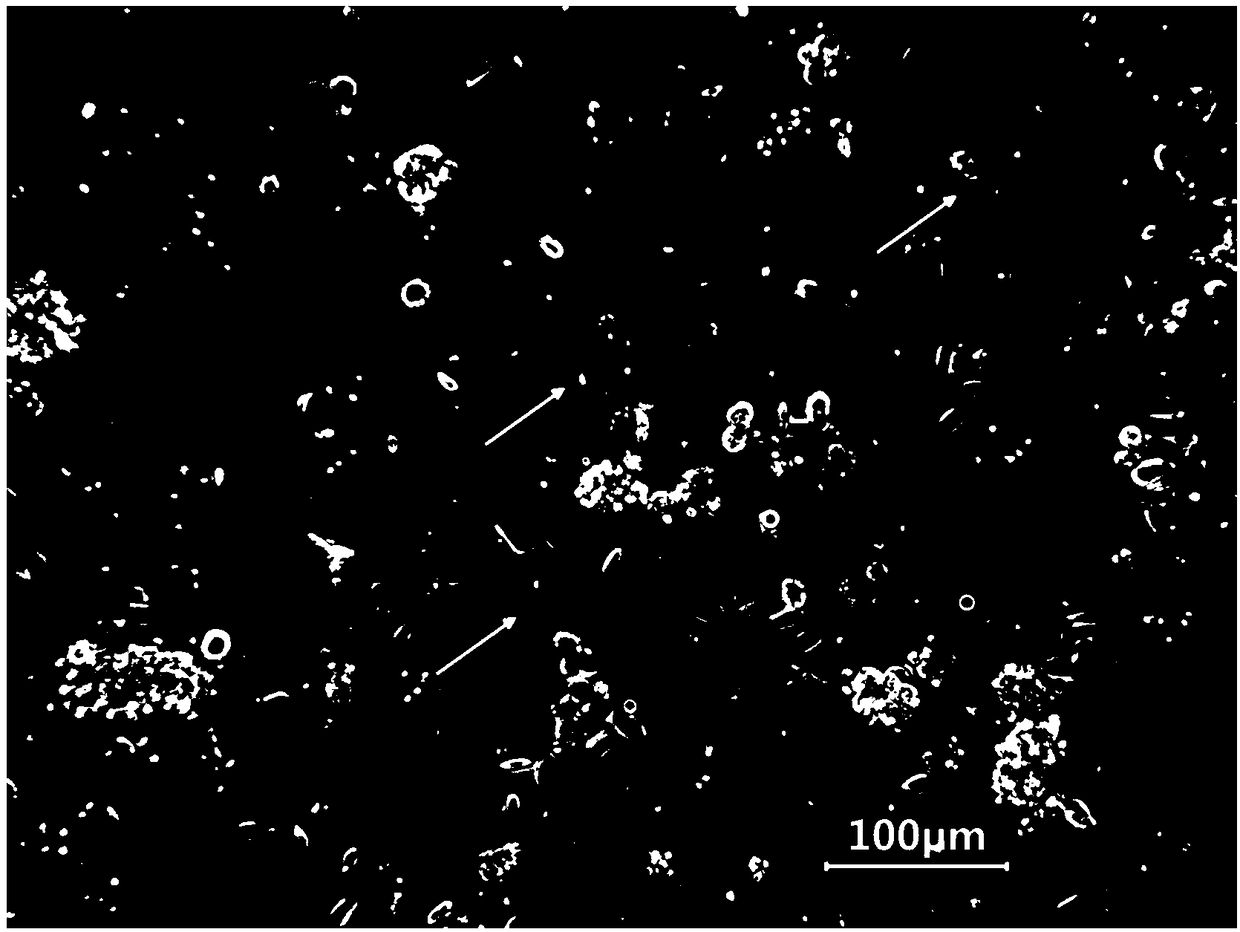
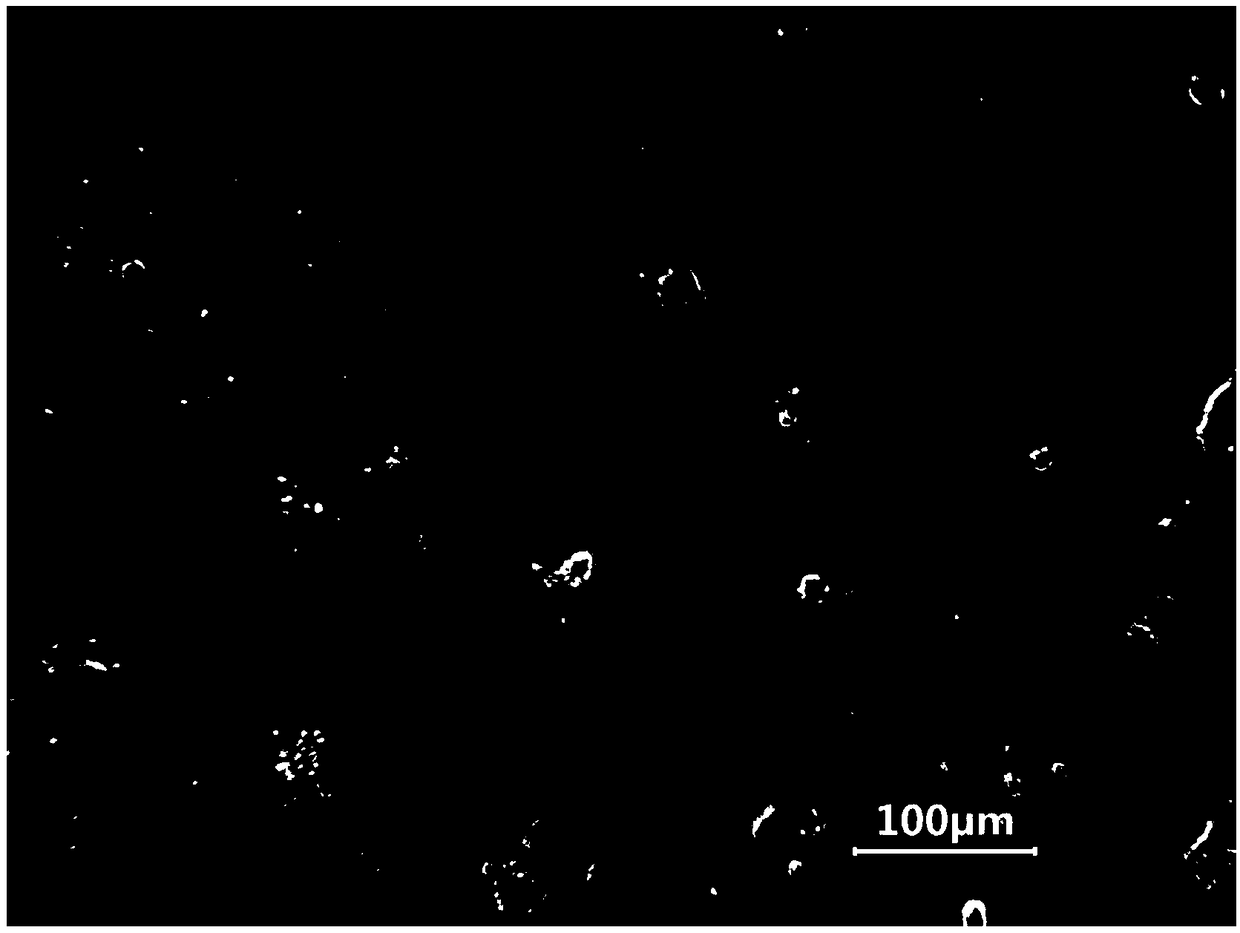
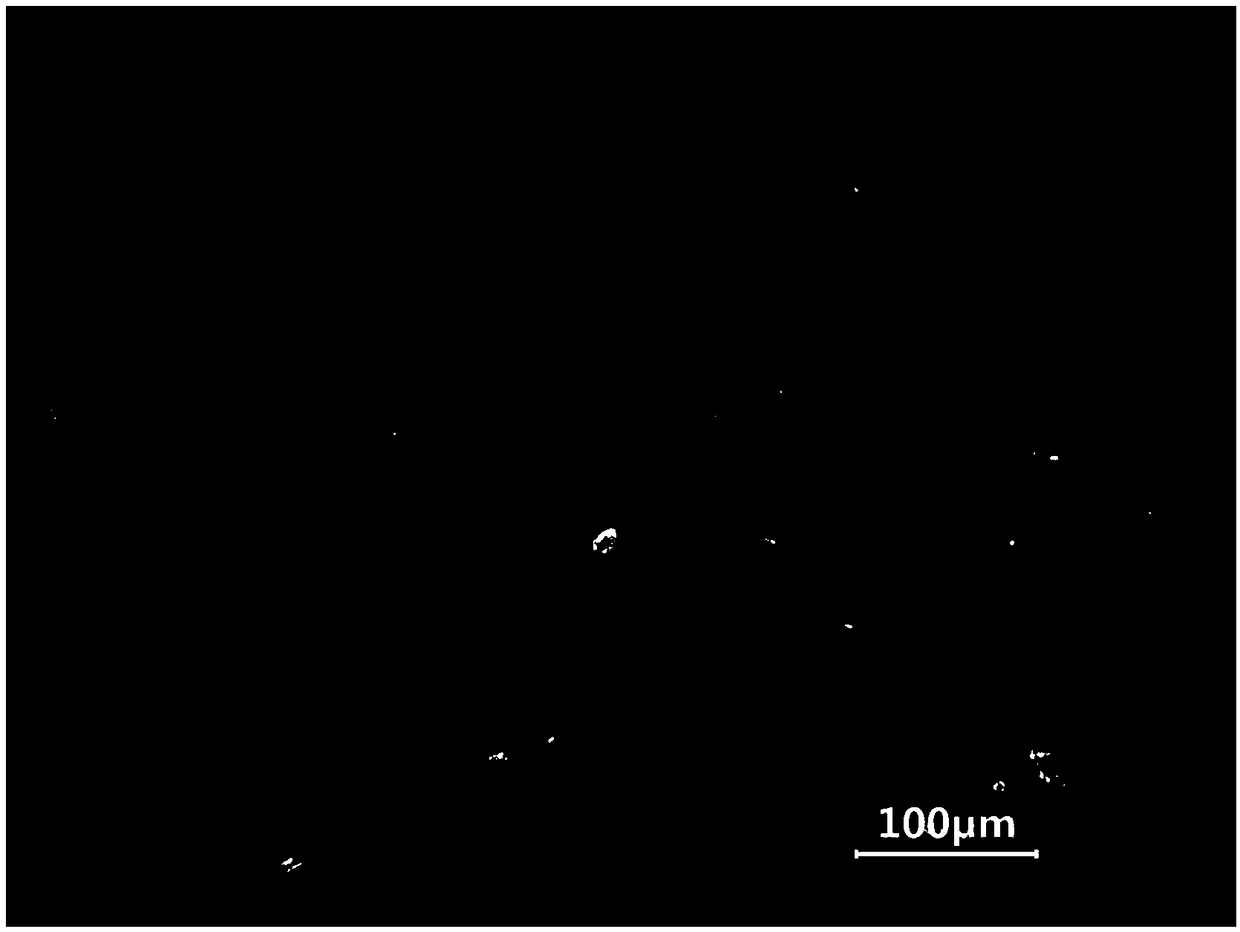
Primary culture method for alveolar epithelial cells of Microhyla ornata
A technology of alveolar epithelium and primary culture, applied in cell dissociation methods, epidermal cells/skin cells, tissue culture and other directions, can solve problems such as blank culture, achieve good growth, simple culture conditions, and less damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 solution
[0041] (1) HBSS balanced salt solution (Hanks’balanced salt solution): take 350mL standard HBSS balanced salt solution (HycloneCat.No.SH30030.02), add 150mL pure water, and store at 4°C for half a year.
[0042](2) Collagenase I solution (collagenase I solution): dissolve 100mg of collagenase I (InvitrogenCat.No.17100-017) in 40mL of HBSS balanced salt solution (see 1), filter through a 0.1μm filter head, and store at -20°C in the dark for one year .
[0043] (3) Y27632 solution: Dissolve 2mg of Y27632 (MCE Cat.No.HY-10583) in 625μL DMSO (SigmaCat.No.D2650), then add 5.625mL of pure water and store at -80°C for half a year.
[0044] (4) L-15 medium stock solution: take 335mL of standard Leibovitz L-15 medium (HycloneCat.No.SH30525.01), add 165mL of pure water, and store at 4°C for half a year.
[0045] (5) Cell complete medium: before use, take 45mL L-15 medium stock solution (see 4), add 5mL fetal bovine serum (FBS) (WISENTC...
Embodiment 2
[0046] Example 2 The primary culture of the alveolar epithelial cells of Rana magnesia
[0047] Go through the following steps:
[0048] (1) Prepare the solution;
[0049] (2) Choose one adult female frog with a body weight of 2.0 grams for freezing anesthesia, rinse with sterile water, and wipe the skin with 75% alcohol cotton ball;
[0050] (3) the frog is placed in a 3.5 cm diameter dish and dissected;
[0051] (4) Cut the lungs with Venus scissors and microscopic tweezers, put them into a new 3.5 cm diameter petri dish, and wash 3 times with HBSS balanced salt solution;
[0052] (5) Use a Pasteur tube to transfer the cleaned lungs into a 15mL centrifuge tube, absorb the residual HBSS balanced salt solution, add 1.5mL collagenase Ⅰ solution, tighten the cap of the centrifuge tube, and digest at 27°C for 5 hours, every 30 minutes Shake the centrifuge tube once;
[0053] (6) Use a Pasteur tube to transfer all the digestive solution (including residual solid lung tissue) i...
Embodiment 3
[0061] Example 3 The primary culture of the alveolar epithelial cells of Rana magnesia
[0062] Go through the following steps:
[0063] (1) Prepare the solution;
[0064] (2) Choose one adult male frog with a body weight of 0.5 grams to freeze and anesthetize, after rinsing with sterile water, wipe the skin with 75% alcohol cotton ball;
[0065] (3) the frog is placed in a 3.5 cm diameter dish and dissected;
[0066] (4) Cut the lungs with Venus scissors and microscopic tweezers, put them into a new 3.5 cm diameter petri dish, and wash 3 times with HBSS balanced salt solution;
[0067] (5) Use a Pasteur tube to transfer the cleaned lungs into a 15mL centrifuge tube, absorb the residual HBSS balanced salt solution, add 1.0mL collagenase Ⅰ solution, tighten the cap of the centrifuge tube, and digest at 27°C for 4 hours, every 30 minutes Shake the centrifuge tube once;
[0068] (6) Use a Pasteur tube to transfer all the digestive solution (including residual solid lung tissu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com