Method for preparing dextrorotatory oxiracetam oral dissolving films by solvent casting
A technology of dextran-olamide and dispersing film, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, nervous system diseases, etc., can solve the problem of low drug loading, difficult control of disintegration time and tensile strength, Restricting the development and application of oral instant film, achieving the effect of simple preparation method, improving bioavailability and avoiding elimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
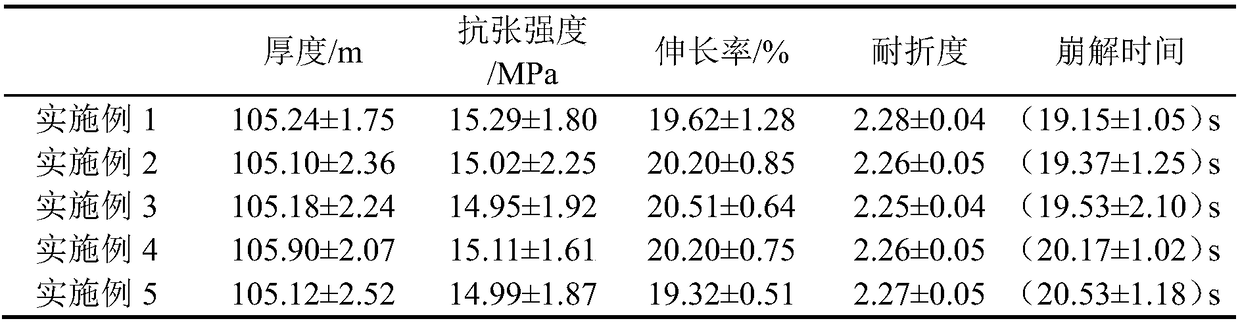
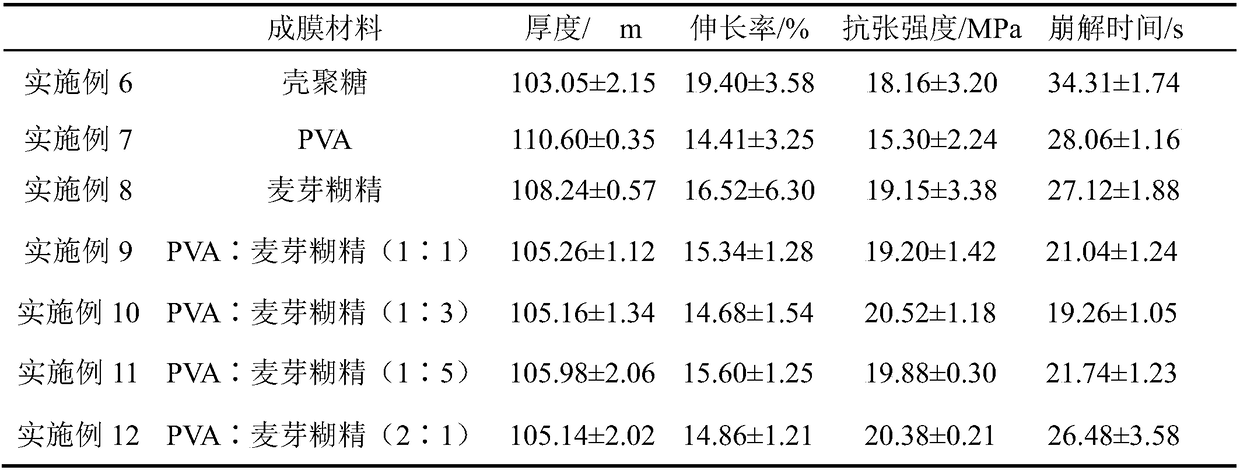
Embodiment 1
[0028] Prescription: 26g dextro-olamide, 18g PVA, 36g maltodextrin, PEG4008g, 9g pregelatinized starch, 2g malic acid, 1g xylitol.
[0029] Preparation:
[0030] (1) Dissolve PVA and maltodextrin in 80mL deionized water to form a homogeneous viscous liquid, then add PEG400 and mix to form material I;
[0031] (2) Mix dexolamide, pregelatinized starch, malic acid and xylitol, and disperse with 50mL of absolute ethanol to form material II;
[0032] (3) Mix the material II with the material I, and stir at a speed of 750r / min to 800r / min for 70-75min to form a viscous suspension of D-olaramide without bubbles;
[0033](4) Cast the D-olaramide non-bubble suspension viscous liquid prepared in step (3) on a mold, dry at 83-85°C, cool at room temperature, form a film, and cut to obtain D-olaramide oral film.
Embodiment 2
[0035] Prescription: 33g of D-olaramide, 12g of PVA, 36g of maltodextrin, 10g of PEG400, 5g of low-substituted hydroxypropyl cellulose, 3g of ascorbic acid, and 1g of fructose.
[0036] Preparation:
[0037] (1) Dissolve PVA and maltodextrin in 70mL deionized water to form a homogeneous viscous liquid, then add PEG400 and mix well to form material I;
[0038] (2) Mix D-olamide, low-substituted hydroxypropyl cellulose, ascorbic acid and fructose, and disperse with 65mL of absolute ethanol to form material II;
[0039] (3) Mix the material II with the material I, and stir at a speed of 500r / min to 550r / min for 95-100min to form a viscous suspension of D-olaramide without bubbles;
[0040] (4) Cast the D-olaramide non-bubble suspension viscous liquid prepared in step (3) on a mold, dry at 65-70°C, cool at room temperature, form a film, and cut to obtain D-olaramide oral film.
Embodiment 3
[0042] Prescription: 40g of dextro-olamide, 15g of PVA, 30g of maltodextrin, 5g of triethyl citrate, 6g of low-substituted hydroxypropyl cellulose, 2g of citric acid, and 1g of glucose.
[0043] Preparation:
[0044] (1) Dissolve PVA and maltodextrin in 50mL deionized water to form a homogeneous viscous liquid, then add triethyl citrate and mix well to form material I;
[0045] (2) Mix D-olamide, low-substituted hydroxypropyl cellulose, citric acid, and glucose, and disperse them with 60mL of absolute ethanol to form material II;
[0046] (3) Mix the material II with the material I, and stir at a speed of 850r / min to 900r / min for 60-65min to form a viscous suspension of D-olaramide without bubbles;
[0047] (4) Cast the D-olaramide non-bubble suspension viscous liquid prepared in step (3) on a mold, dry at 90-95°C, cool at room temperature, form a film, and cut to obtain D-olaramide oral film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com