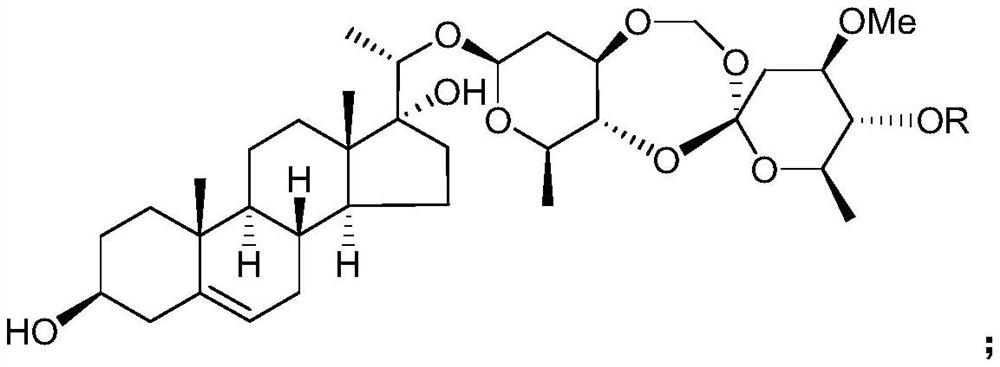
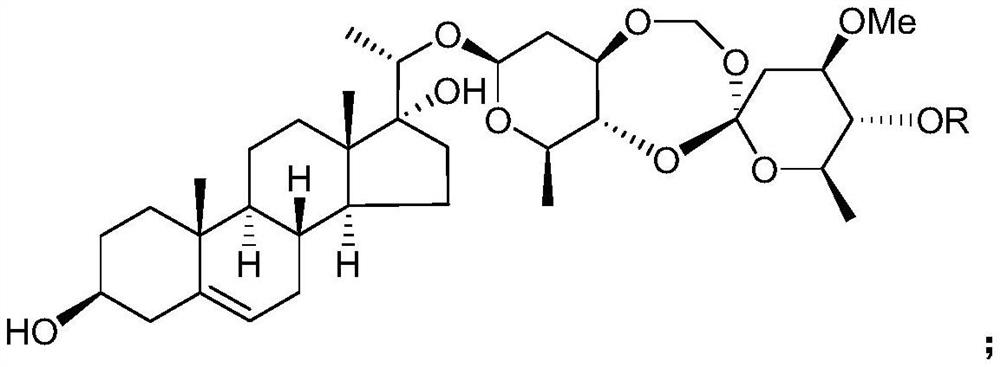
The use of the C21 steroid compound in the preparation of IDO inhibitor
The technology of a kind of black bone vine and compound, applied in the field of preparation of IDO inhibitor, black bone vine C21 steroid compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of C21 steroids from Heliconia chinensis
[0033] Get the root of Teng Teng, pulverize it, add 3 times the weight of ethanol aqueous solution with a volume concentration of 95% to extract twice at room temperature, extract for 7 days each time, combine the extracts, concentrate under reduced pressure until there is no alcohol smell, and obtain Teng Teng extract;
[0034] suspending the extract of Rhizoma chinensis in water of 1 times the weight, then extracting twice with chloroform as the extractant, collecting the organic phase of the extract, and then concentrating under reduced pressure to obtain the extract of the chloroform extraction part of Rhizoma Rhizoma;
[0035] Take the extract from the chloroform extraction part of Rhododendron chinensis and purify it by silica gel column chromatography, using petroleum ether as mobile phase A and acetone as mobile phase B, and carry out gradient elution according to the following procedure: first u...
experiment example 1
[0038] Experimental example 1 Study on the inhibitory activity of the C21 steroid compound of the present invention on IDO
[0039] 1. Purpose of the experiment
[0040] The plasmid pcDNA3.1-IDO was used to transfect HEK293 cells to make it highly express IDO, and then the inhibitory activity of the C21 steroid compound of the present invention on IDO was determined at the cellular level.
[0041] 2. Experimental method
[0042] HEK 293 cells were seeded in a 96-well plate at a density of 2.5X104 cells / well, cultured in DMEM medium (containing 10% fetal bovine serum, 50 U / mL penicillin and 50 mg / mL streptomycin), placed at 37 ° C, humidity 95%, 5% CO 2 cultured in an incubator. After culturing for 24 hours, use liposome Lipofectamin 2000 to mediate pcDNA3.1-hIDO plasmid transfection, and divide them into positive control group and experimental group 1-2.
[0043] The positive control group used 1-methyltryptophan (1-MT) as the test substance, and the experimental group ...
experiment example 2
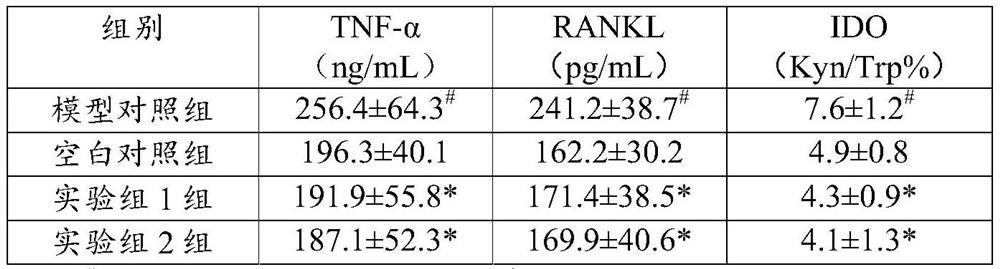
[0053] Experimental example 2 Therapeutic effect of the C21 steroid compound of the present invention on ankylosing spondylitis
[0054] 1. Purpose of the experiment
[0055] The mouse model of ankylosing spondylitis was established by proteoglycan immunization method, and then the C21 steroid compound of the present invention was gavaged, and the inflammatory marker serum TNF-α and NF-кB receptor activator ligand ( RANKL) level, and detect serum IDO activity (Kyn / Trp), verify the curative effect of C21 steroid compound of the present invention for ankylosing spondylitis.
[0056] 2. Experimental method
[0057] 2.1 Experimental animals
[0058] Thirty-two healthy male BALB / c mice, weighing (18±2) g, aged 4-5 weeks, were purchased from Shanghai SLAC.
[0059] 2.2 Drugs to be tested
[0060] The saccharidin L and saccharidin H prepared in Example 1 were used as the test drugs.
[0061] 2.3 Experimental grouping and modeling
[0062] After the mice were adaptively fed f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com