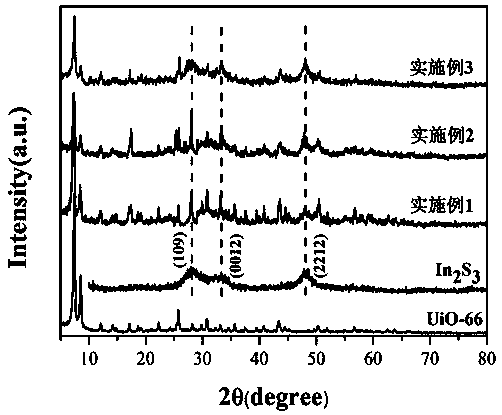
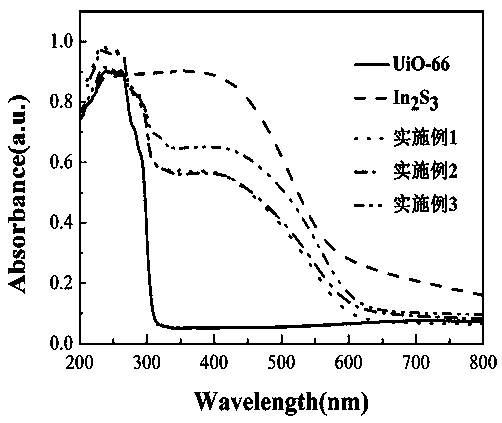
Preparation method of core-shell structure visible-light-driven photocatalyst
A core-shell structure and visible light technology, applied in the field of photocatalysis, can solve the problems of harsh preparation conditions, complicated preparation process, and limited catalytic effect, and achieve the effects of improved photocatalytic ability, high mobility, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) According to ZrCl 4 and terephthalic acid (H 2 BDC) with a molar ratio of 1:1, respectively weighed ZrCl 4 and terephthalic acid, dispersed in N,N-dimethylformamide, then, according to the volume ratio of N,N-dimethylformamide and acetic acid is 10:1, add acetic acid, stir for 30min and move to In the microwave reactor, the reaction temperature is 120° C., and the reaction time is 0.5 h. Cool to room temperature after the reaction, filter with suction, wash with N,N-dimethylformamide and deionized water three times alternately, dry and grind to obtain the UiO-66 matrix material.
[0020] 2) Disperse UiO-66 in deionized water, according to In(NO 3 ) 3 • 5H 2 In(NO 3 ) 3 • 5H 2 O was added to the aqueous dispersion of UiO-66, and after stirring for 30 min, according to dodecylamine, sulfur, sodium lignosulfonate and In(NO 3 ) 3 • 5H 2 The molar ratio of O is 0.1:1.5:0.1:1 and the above substances are added in sequence, and the pH of the system is adjusted to...
Embodiment 2
[0026] 1) According to ZrCl 4 and terephthalic acid (H 2 The molar ratio of BDC) is 1~1, respectively weigh ZrCl 4 and terephthalic acid, dispersed in N,N-dimethylformamide, then, according to the volume ratio of N,N-dimethylformamide and acetic acid is 10~11:1, add acetic acid, after stirring for 30min Move to a microwave reactor, the reaction temperature is 140°C, and the reaction time is 1h. Cool to room temperature after the reaction, filter with suction, wash with N,N-dimethylformamide and deionized water three times alternately, dry and grind to obtain the UiO-66 matrix material.
[0027] 2) Disperse UiO-66 in deionized water, according to In(NO 3 ) 3 • 5H 2 In(NO 3 ) 3 • 5H 2 O was added to the aqueous dispersion of UiO-66, and after stirring, according to dodecylamine, sulfur, sodium lignosulfonate and In(NO 3 ) 3 • 5H 2 The molar ratio of O is 0.2:2:0.2:1, and the above substances are added in sequence, and the pH of the system is adjusted to 3 with 0.5mol / ...
Embodiment 3
[0034] 1) According to ZrCl 4 and terephthalic acid (H 2 The molar ratio of BDC) is 1:1.2, respectively weigh ZrCl 4 and terephthalic acid, dispersed in N,N-dimethylformamide, then, according to the volume ratio of N,N-dimethylformamide and acetic acid is 12:1, add acetic acid, stir and move to microwave In the reaction kettle, the reaction temperature is 160° C., and the reaction time is 1.5 h. Cool to room temperature after the reaction, filter with suction, wash with N,N-dimethylformamide and deionized water three times alternately, dry and grind to obtain the UiO-66 matrix material.
[0035] 2) Disperse UiO-66 in deionized water, according to In(NO 3 ) 3 • 5H 2 In(NO 3 ) 3 • 5H 2 O was added to the aqueous dispersion of UiO-66, and after stirring, according to dodecylamine, sulfur, sodium lignosulfonate and In(NO 3 ) 3 • 5H 2 The molar ratio of O is 0.3:3:0.3:1, and the above substances are added in sequence, and the pH of the system is adjusted to 3 with 0.5mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com