Method for preparing lithium-enriched solution and manganese dioxide by utilizing failed lithium manganese phosphate
A technology of lithium manganese phosphate and manganese dioxide, applied in manganese oxide/manganese hydroxide, recycling technology, lithium carbonate;/acid carbonate, etc., can solve problems such as limiting battery energy density, and achieve shortening Cleaning time, achieve recycling, avoid pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
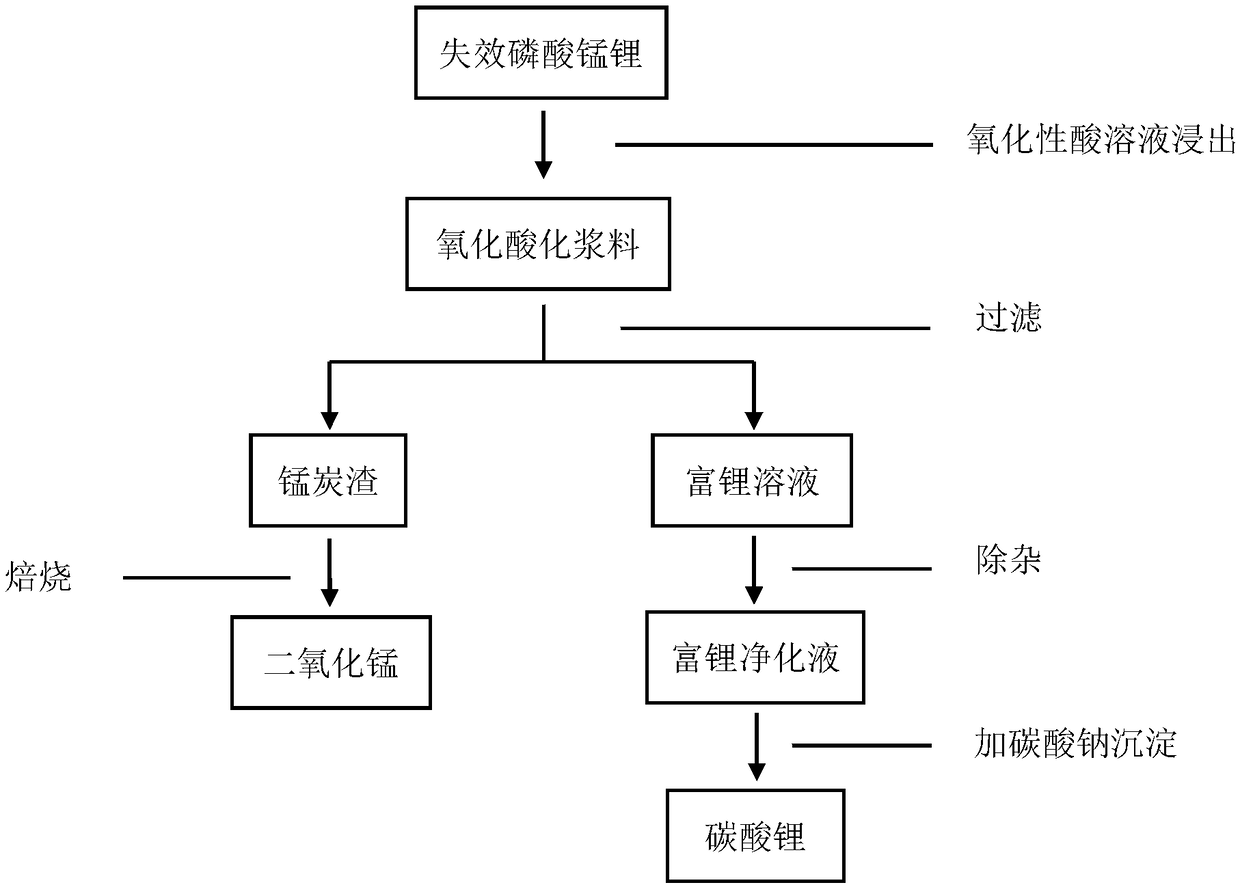
[0022] Such as figure 1 As shown, first dissolving the invalid lithium manganese phosphate in the sodium persulfate solution, the oxidative acidification slurry is obtained through the oxidation reaction; then the lithium-rich solution and the manganese carbon residue are obtained through filtration, and the manganese carbon residue is a mixture of manganese dioxide and carbon; The lithium-rich solution is purified to obtain a lithium-rich purification solution, and the lithium-rich purification solution is precipitated with a sodium carbonate solution to obtain lithium carbonate; the manganese carbon residue is roasted at 750°C in an oxygen atmosphere to obtain manganese dioxide. The impurity removal operation is to use sodium hydroxide to adjust the pH value of the lithium-rich solution to 8 to remove a small amount of manganese ions, and then use sodium hydroxide to adjust the pH value to 11.
Embodiment 2
[0024] Such as figure 1 As shown, first dissolving the invalid lithium manganese phosphate in the sodium persulfate solution, the oxidative acidification slurry is obtained through the oxidation reaction; then the lithium-rich solution and the manganese carbon residue are obtained through filtration, and the manganese carbon residue is a mixture of manganese dioxide and carbon; The lithium-rich solution is purified to obtain a lithium-rich purified solution, and the lithium-rich purified solution is precipitated with a sodium carbonate solution to obtain lithium carbonate; the manganese carbon residue is roasted at 700°C in an oxygen atmosphere to obtain manganese dioxide. The impurity removal operation is to use sodium hydroxide to adjust the pH value of the lithium-rich solution to 8 to remove a small amount of manganese ions, and then use sodium hydroxide to adjust the pH value to 11.
Embodiment 3
[0026] Such as figure 1 As shown, first dissolving the invalid lithium manganese phosphate in the sodium persulfate solution, the oxidative acidification slurry is obtained through the oxidation reaction; then the lithium-rich solution and the manganese carbon residue are obtained through filtration, and the manganese carbon residue is a mixture of manganese dioxide and carbon; The lithium-rich solution is purified to obtain a lithium-rich purified solution, and the lithium-rich purified solution is precipitated with a sodium carbonate solution to obtain lithium carbonate; the manganese carbon residue is roasted at 700°C in an oxygen atmosphere to obtain manganese dioxide. The impurity removal operation is to use sodium hydroxide to adjust the pH value of the lithium-rich solution to 8 to remove a small amount of manganese ions, and then use sodium hydroxide to adjust the pH value to 11.
[0027] The oxidation reaction is completed in a stainless steel container. After the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com