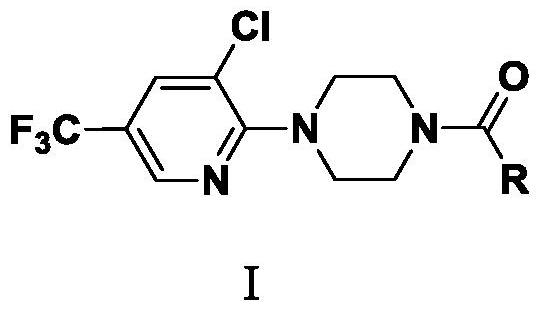
A kind of fluorine-containing pyridine piperazine amide compound and its application
A technique for pyridine piperazine amides and compounds, which is applied in the field of fluorine-containing pyridine piperazine amides to achieve the effects of high drug efficacy, simple synthesis process and good biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
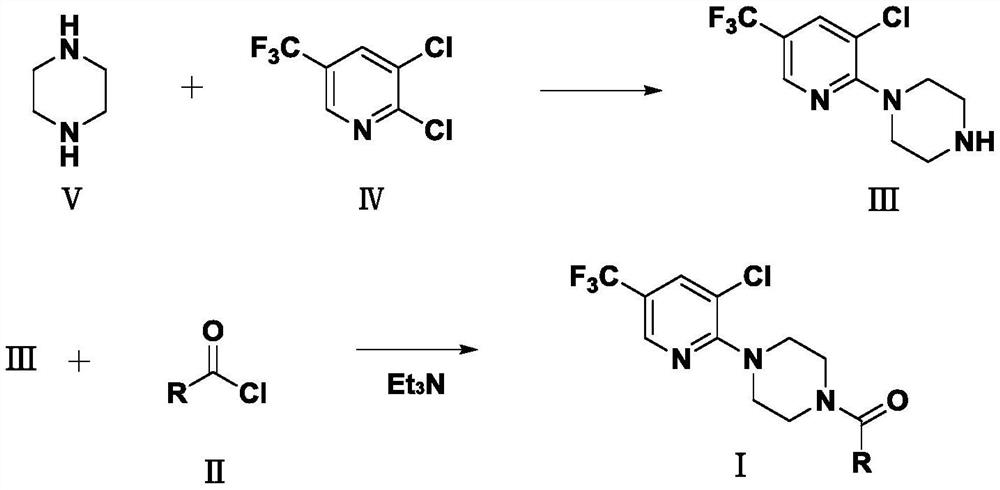
[0024] Example 1, the preparation of intermediate III
[0025]
[0026] Add 17.2g (0.2mol) of anhydrous piperazine and 60mL of methanol into a 250mL reaction flask, stir to dissolve and raise the temperature to 40°C, then dilute 21.6g (0.1mol) of 2,3-dichloro-5-(tri Fluoromethyl)pyridine, slowly added dropwise, after the drop, reacted at a constant temperature of 40°C for 6h, tracked by TLC until the reaction of the raw materials was complete, lowered to room temperature, filtered with suction, and the filtrate was rotary evaporated in vacuo to remove methanol, and a white solid was precipitated. Add water and stir to dissolve the remaining free Water piperazine, filtered with suction, and washed the filter cake with water to obtain 22.6 g of a white solid with a melting point of 65.8-67.3°C and a yield of 85%.
[0027] 1 H NMR (500MHz, DMSO-d6), δ (ppm) 8.53 (s, 1H), 8.15 (s, 1H), 3.37–3.35 (t, 4H), 2.82–2.80 (t, 4H).
example 2
[0028] Example 2, compound I 1 preparation of
[0029]
[0030] Take 5.3g (0.02mol) of intermediate III and add it to a 250mL reaction flask, then add 35mL of ethyl acetate, then add 2.22g of triethylamine, add 10mL of ethyl acetate and 3.45g (0.02mol) of 3-ethylamine dropwise under stirring - Mixture of 1-methyl-1H-pyrazole-5-carbonyl chloride, react at room temperature for 30 minutes after dropping, TLC traces the complete reaction of intermediate III, add 100mL of water and stir, then separate the liquids, and dry the organic phase with anhydrous sodium sulfate , ethyl acetate was removed by rotary evaporation, and 7.3 g of white solid was obtained by cooling, with a melting point of 103.0-105.1° C. and a yield of 91%.
[0031] 1 H NMR (500MHz, DMSO-d6) δ (ppm) 8.56 (s, 1H), 8.23 (s, 1H), 6.38 (s, 1H), 3.97 (s, 2H), 3.82 (s, 3H), 3.58– 3.44(m,6H),2.57–2.51(m,2H),1.24–1.21(t,3H).
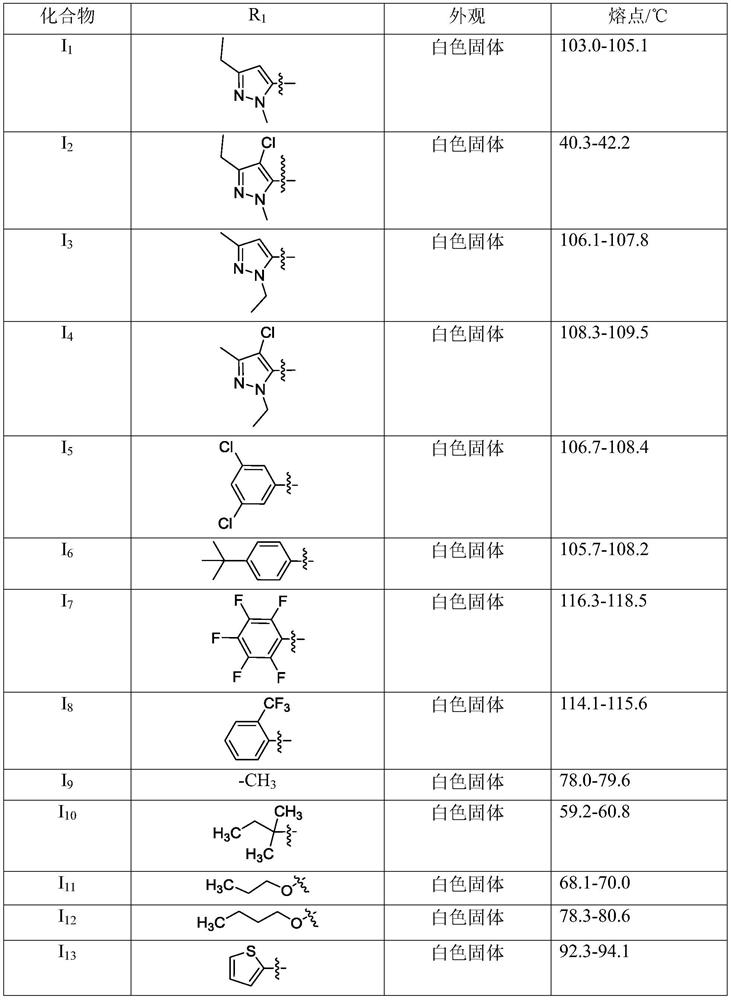
[0032] According to example 2 in I 1 Synthetic method for synthesizing other compoun...
example 3
[0046] Example 3, acaricidal activity assay
[0047] The acaricidal egg activity was determined by dipping method and statistical correction of pest mortality. The specific process is as follows: dilute the test agent to the required concentration according to the active ingredients, draw 50mL of the liquid medicine under aseptic conditions and inject it into the petri dish, and then respectively immerse the cinnabar mite eggs (mite eggs normally raised according to the indoor standardized method) population), and a plate with 50 mL of sterilized water added was used as a blank control. Place the Petri dish in a constant temperature incubator at 24±1°C. After 48 hours, the mortality rate was investigated and counted.
[0048] The acaricidal activity test result of table 1 series compound
[0049]
[0050] According to the test results, the compound of the present invention has excellent acaricidal activity, and at the same dose, the compound of the present invention I 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com