Synthesis method and application of rosin-based imidazoline derivative corrosion inhibitor
A technology of rosin-based imidazoline and synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. energy and other problems, to achieve the effect of reducing the degree of pitting corrosion, simple preparation method and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
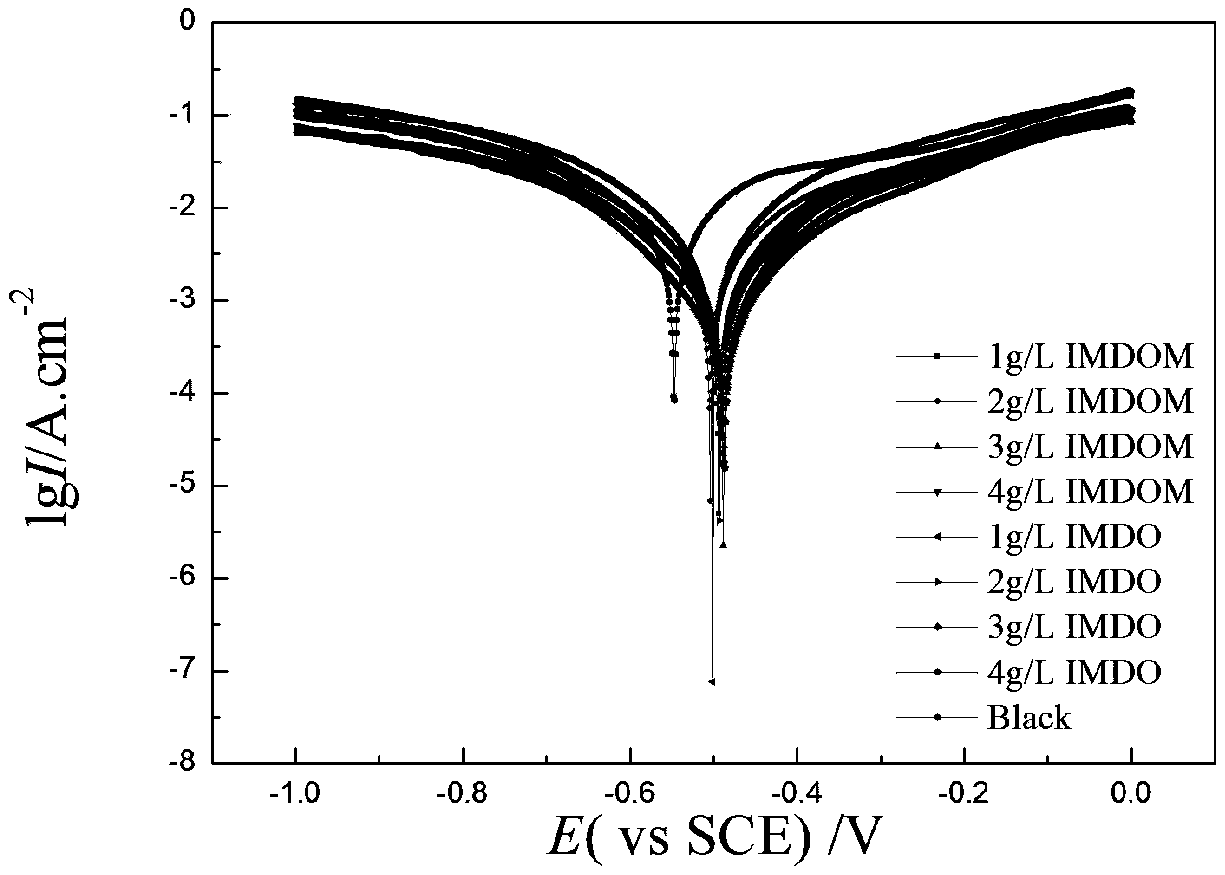
Examples
Embodiment 1
[0054] (1) Preparation of corrosion inhibitor intermediate
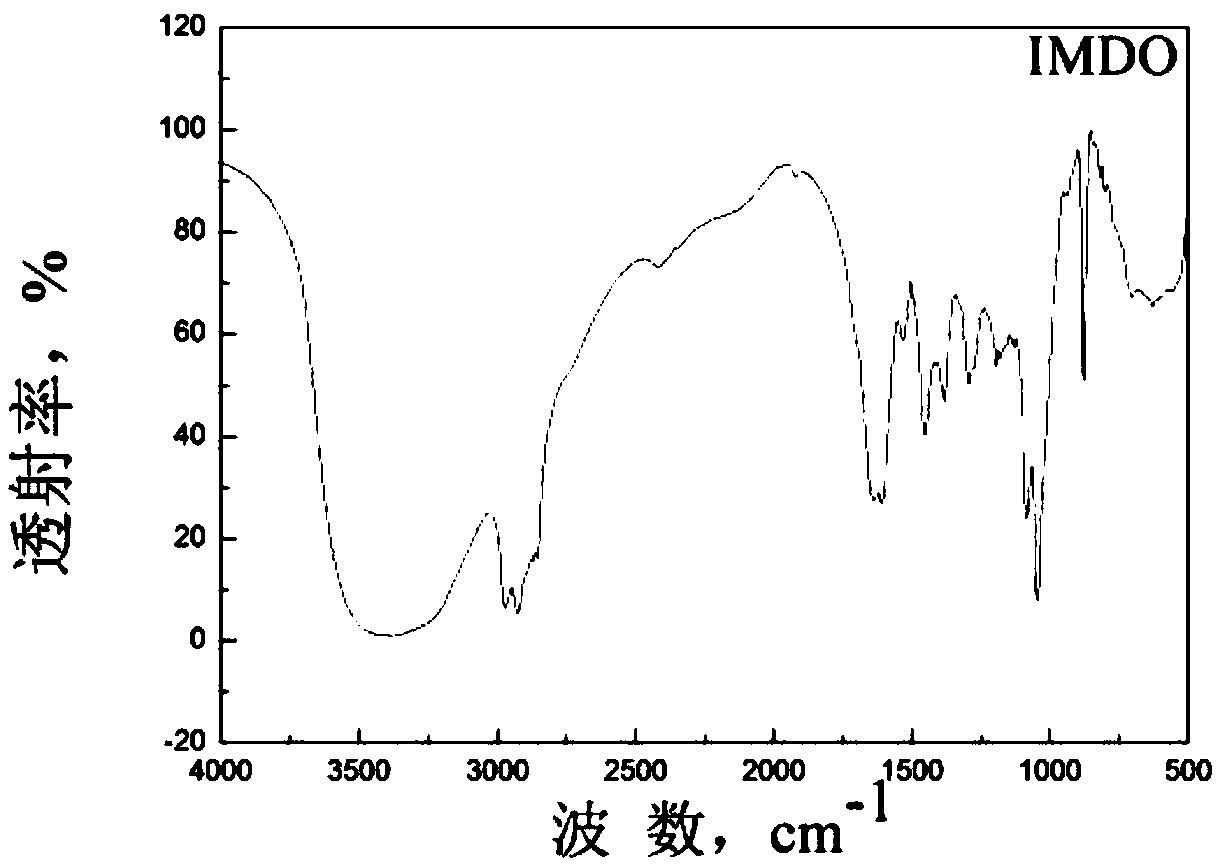
[0055] Add dehydroabietic acid, triethylenetetramine and xylene in a molar ratio of 1:1.1:0.8, first add dehydroabietic acid into a dry four-necked bottle equipped with a water separator, and raise the temperature to 220-240°C. Slowly add triethylenetetramine and xylene as water-carrying agents dropwise, react for 3-6 hours; then continue to heat up to 270-280°C, reflux for cyclization reaction for 3-6 hours, and use a water separator to separate water; finally distill out under reduced pressure Xylene, obtains the rosin-based imidazoline derivative corrosion inhibitor intermediate IMDO;
[0056] (2) Mannich reaction
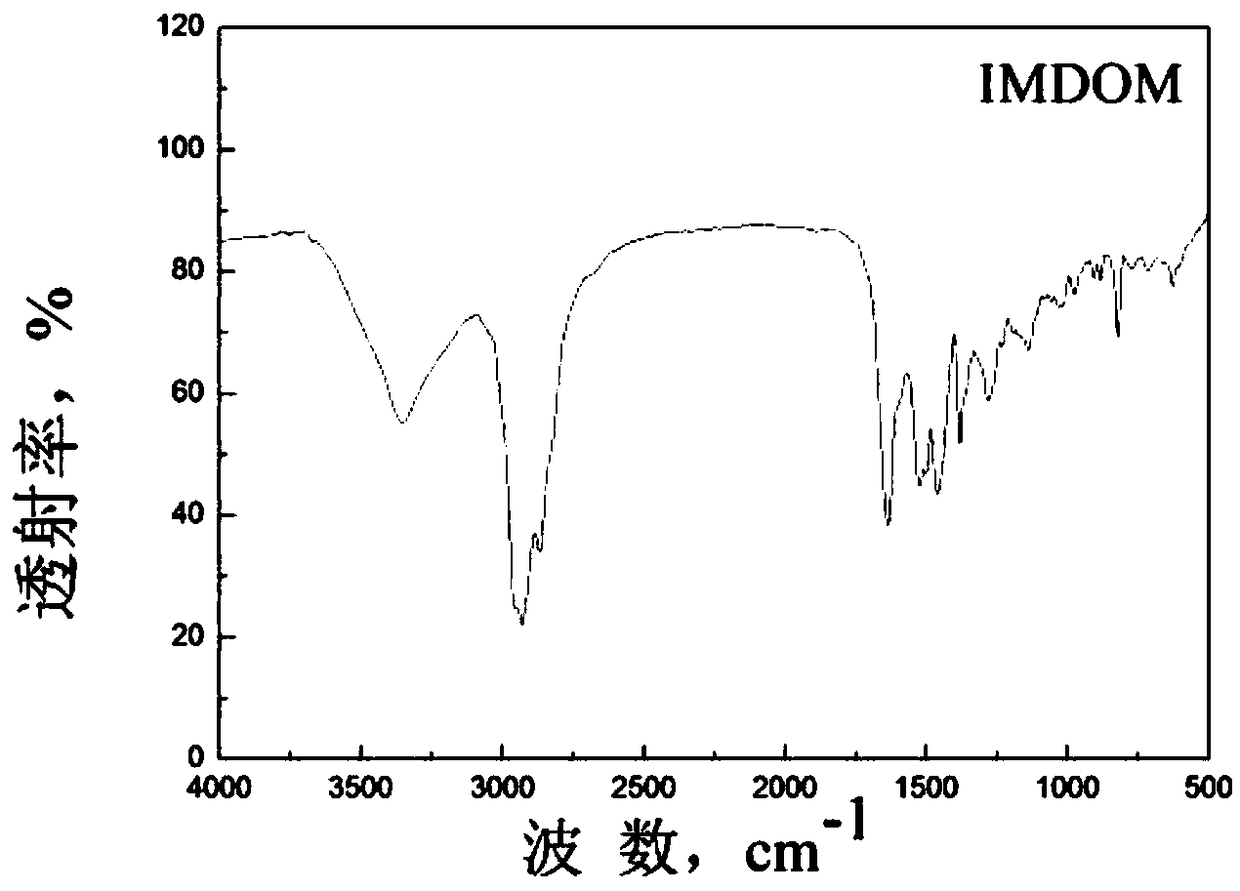
[0057] h 3 PO 3 The molar ratio of intermediate IMDO and formaldehyde is 1:1:2. In an acidic catalytic environment, first add intermediate IMDO and phosphorous acid and heat at reflux at 100-110°C for 1.5-2.5h, then slowly add formaldehyde dropwise with a constant pressure funnel , reflux reaction ...
Embodiment 2
[0059] (1) Preparation of corrosion inhibitor intermediate
[0060] Add dehydroabietic acid, triethylenetetramine and xylene in a molar ratio of 1:1.3:1.1, first add dehydroabietic acid into a dry four-necked bottle equipped with a water separator, and raise the temperature to 220-240°C. Slowly add triethylenetetramine and xylene as water-carrying agents dropwise, react for 3-6 hours; then continue to heat up to 270-280°C, reflux for cyclization reaction for 3-6 hours, and use a water separator to separate water; finally distill out under reduced pressure Xylene, obtains the rosin-based imidazoline derivative corrosion inhibitor intermediate IMDO;
[0061] (2) Mannich reaction
[0062] h 3 PO 3 The molar ratio of intermediate IMDO and formaldehyde is 1:1:2. In an acidic catalytic environment, first add intermediate IMDO and phosphorous acid and heat at reflux at 100-110°C for 1.5-2.5h, then slowly add formaldehyde dropwise with a constant pressure funnel , reflux reaction ...
Embodiment 3
[0064] (1) Preparation of corrosion inhibitor intermediate
[0065] Add dehydroabietic acid, triethylenetetramine and xylene in a molar ratio of 1:1.2:0.9, first add dehydroabietic acid into a dry four-necked bottle equipped with a water separator, and raise the temperature to 220-240°C. Slowly add triethylenetetramine and xylene as water-carrying agents dropwise, react for 3-6 hours; then continue to heat up to 270-280°C, reflux for cyclization reaction for 3-6 hours, and use a water separator to separate water; finally distill out under reduced pressure Xylene, obtains the rosin-based imidazoline derivative corrosion inhibitor intermediate IMDO;
[0066] (2) Mannich reaction
[0067] h 3 PO 3 The molar ratio of intermediate IMDO and formaldehyde is 1:1:2. In an acidic catalytic environment, first add intermediate IMDO and phosphorous acid and heat at reflux at 100-110°C for 1.5-2.5h, then slowly add formaldehyde dropwise with a constant pressure funnel , reflux reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com