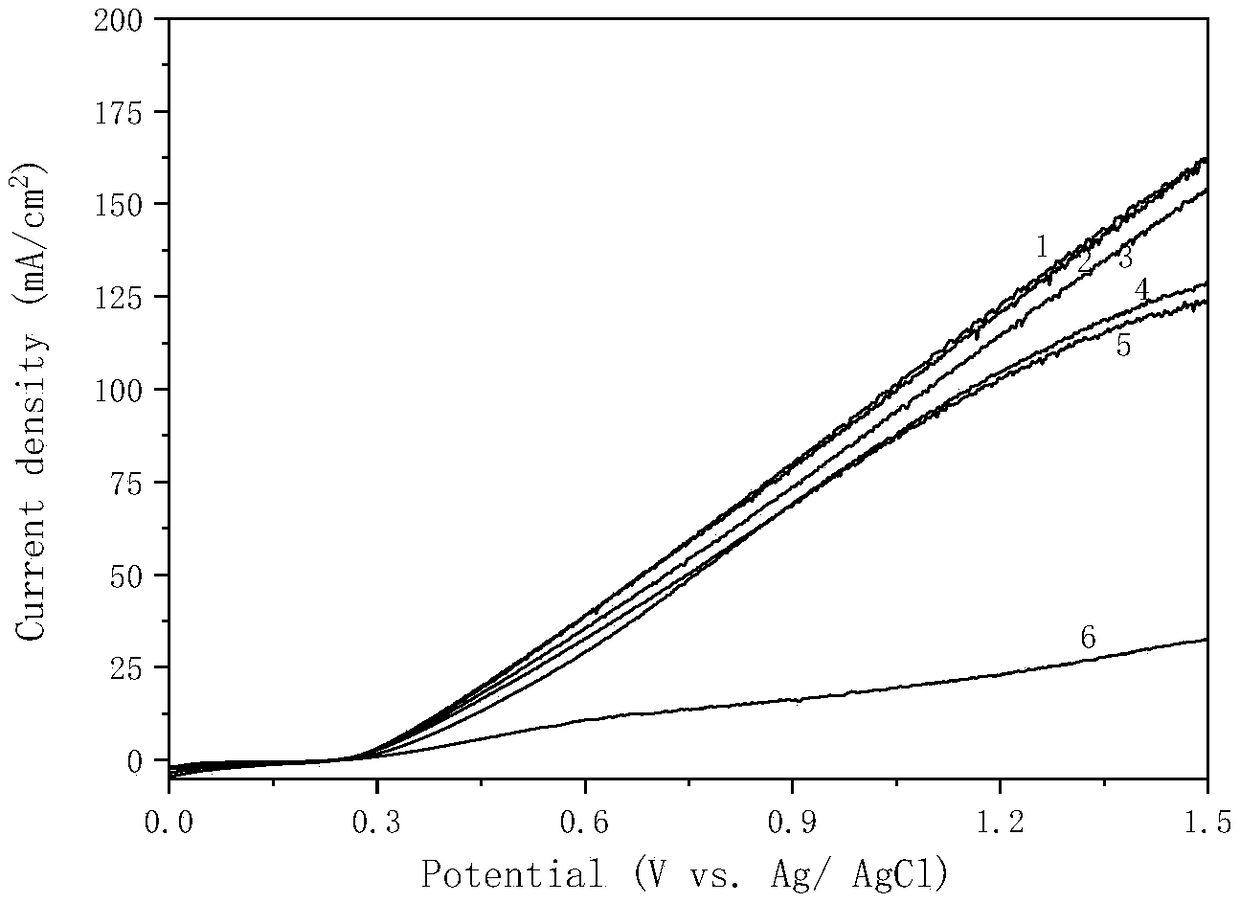
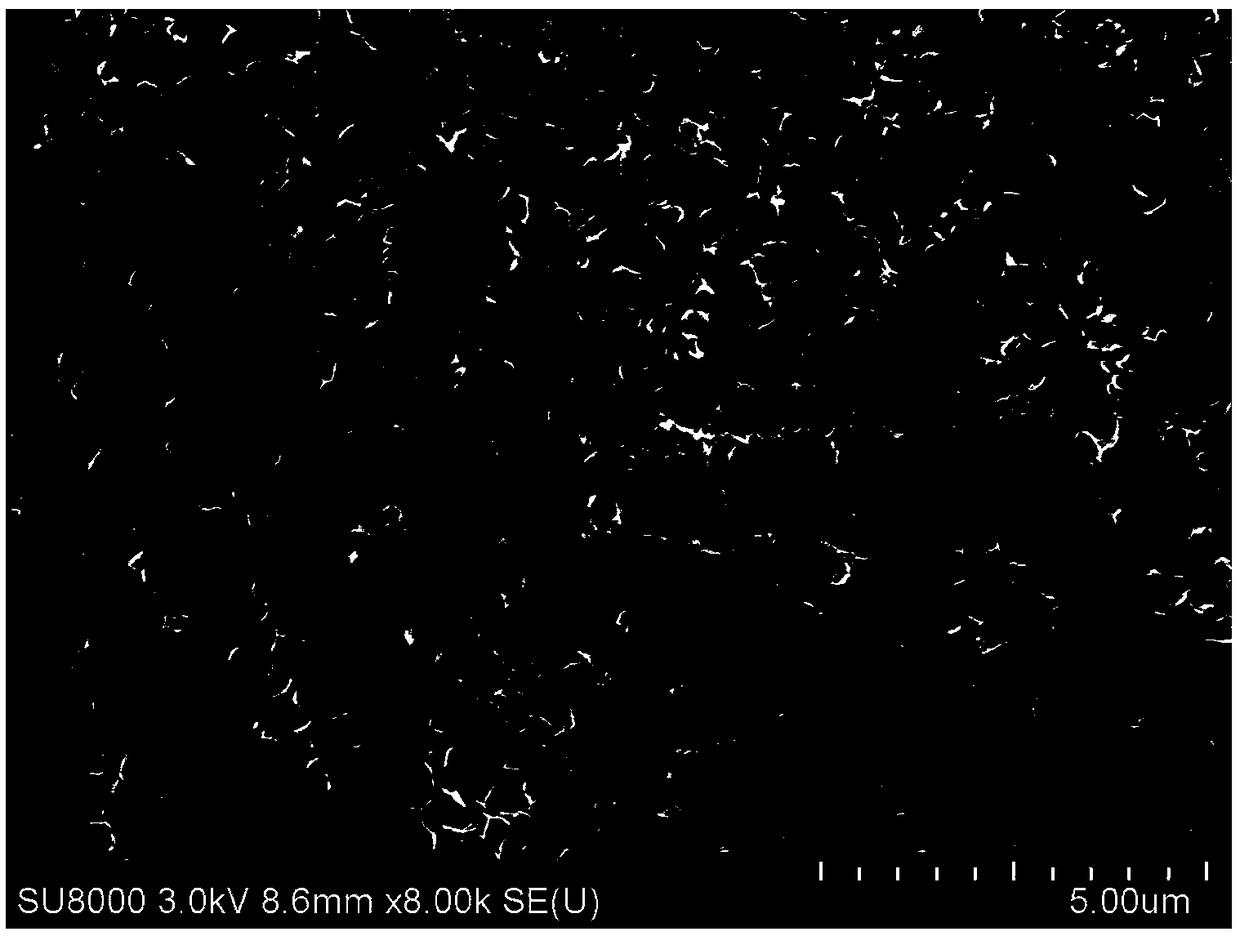
Titanium sheet with surface modified sulfur doped titanium dioxide nanoflakes as well as preparation method and application of titanium sheet
A titanium dioxide and surface modification technology, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of poor electrical conductivity and strong acid resistance, and achieve good stability and good electrical conductivity , the effect of high electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Titanium sheet surface treatment
[0041] The cut titanium sheet (2×3cm) was ultrasonically treated with acetone, distilled water and ethanol for 30 minutes respectively, and the treated titanium sheet was air-dried.
[0042] (2) Preparation of mixed solution
[0043] Dissolve 1g of urea and 0.25g of TAA in a mixed solution of 15mL of distilled water and 15mL of ethanol, and stir magnetically at room temperature until the urea and TAA are evenly dispersed to obtain a mixed solution of urea and TAA (the concentration of urea is 0.56mol / L, the concentration of TAA The concentration is 0.111mol / L).
[0044] (3) Modification of the surface of the titanium sheet
[0045] The titanium sheet after step (1) has been processed leans against the inner wall of the polytetrafluoroethylene kettle lining, and then the mixed solution obtained by step (2) preparation is transferred to the polytetrafluoroethylene kettle lining of 50mL, Install the reaction kettle and react in an ...
Embodiment 2
[0047] (1) Titanium sheet surface treatment
[0048] The cut titanium sheet (2×3cm) was ultrasonically treated with acetone, distilled water and ethanol for 30 minutes respectively, and the treated titanium sheet was air-dried.
[0049] (2) Preparation of mixed solution
[0050] Dissolve 1g of urea and 0.25g of TAA in a mixed solution of 15mL of distilled water and 15mL of ethanol, and stir magnetically at room temperature until the urea and TAA are evenly dispersed to obtain a mixed solution of urea and TAA (the concentration of urea is 0.56mol / L, the concentration of TAA The concentration is 0.111mol / L).
[0051] (3) Modification of the surface of the titanium sheet
[0052] The titanium sheet after step (1) has been processed leans against the inner wall of the polytetrafluoroethylene kettle lining, and then the mixed solution obtained by step (2) preparation is transferred to the polytetrafluoroethylene kettle lining of 50mL, Install the reaction kettle and react in an ...
Embodiment 3
[0054] (1) Titanium sheet surface treatment
[0055] The cut titanium sheet (2×3cm) was ultrasonically treated with acetone, distilled water and ethanol for 30 minutes respectively, and the treated titanium sheet was air-dried.
[0056] (2) Preparation of mixed solution
[0057] Dissolve 1g of urea and 0.25g of TAA in a mixed solution of 15mL of distilled water and 15mL of ethanol, and stir magnetically at room temperature until the urea and TAA are evenly dispersed to obtain a mixed solution of urea and TAA (the concentration of urea is 0.56mol / L, the concentration of TAA The concentration is 0.111mol / L).
[0058] (3) Modification of the surface of the titanium sheet
[0059] The titanium sheet after step (1) has been processed leans against the inner wall of the polytetrafluoroethylene kettle lining, and then the mixed solution obtained by step (2) preparation is transferred to the polytetrafluoroethylene kettle lining of 50mL, Install the reaction kettle and react in an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com