Asymmetric seven-membered fused thiophene as well as preparation method and application thereof
A fused thiophene, asymmetric technology, applied in the field of preparation of organic compounds, can solve the problems of affecting the intermolecular stacking mode, affecting the optoelectronic properties of materials, etc., and achieve the effect of high yield and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment provides a preparation method of asymmetric heptathiophene substituted with double TMS, including the following steps:
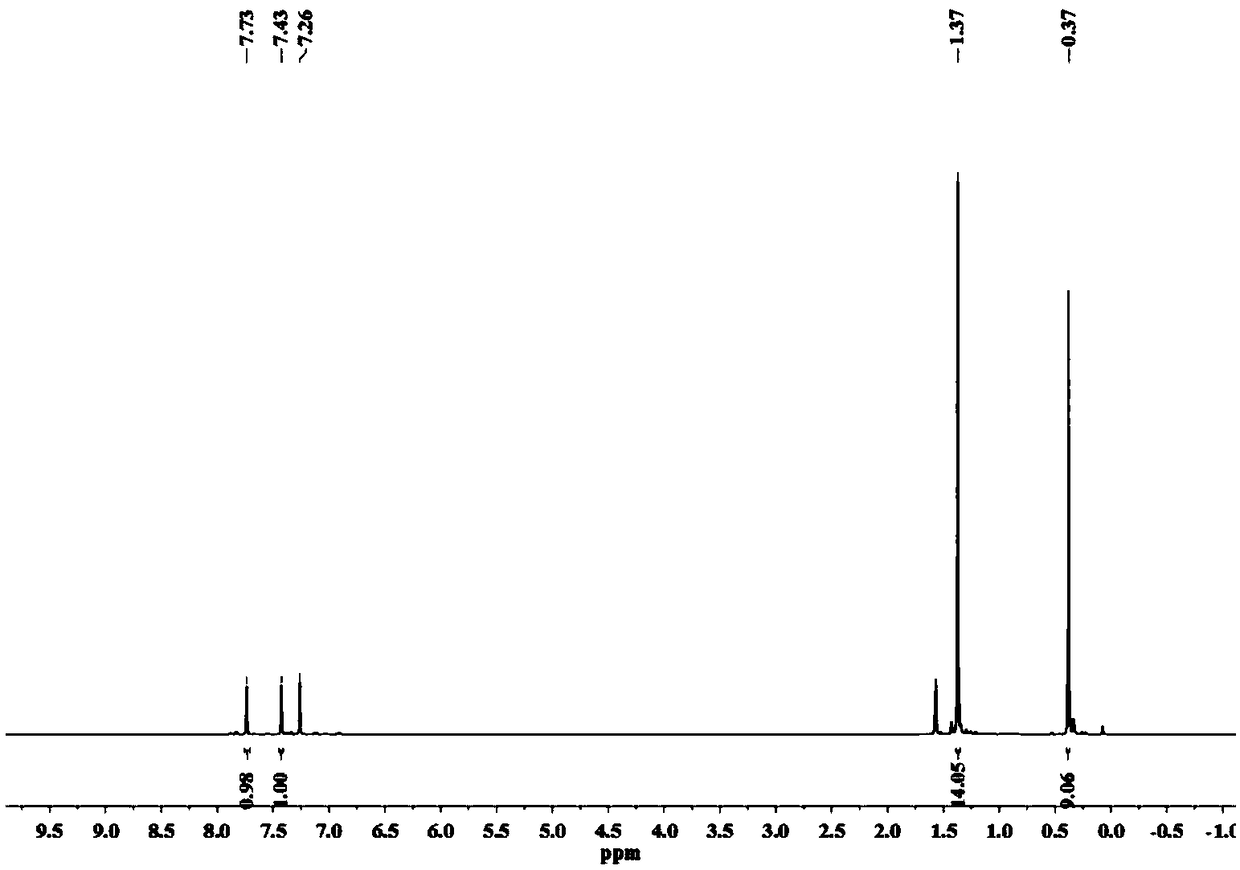
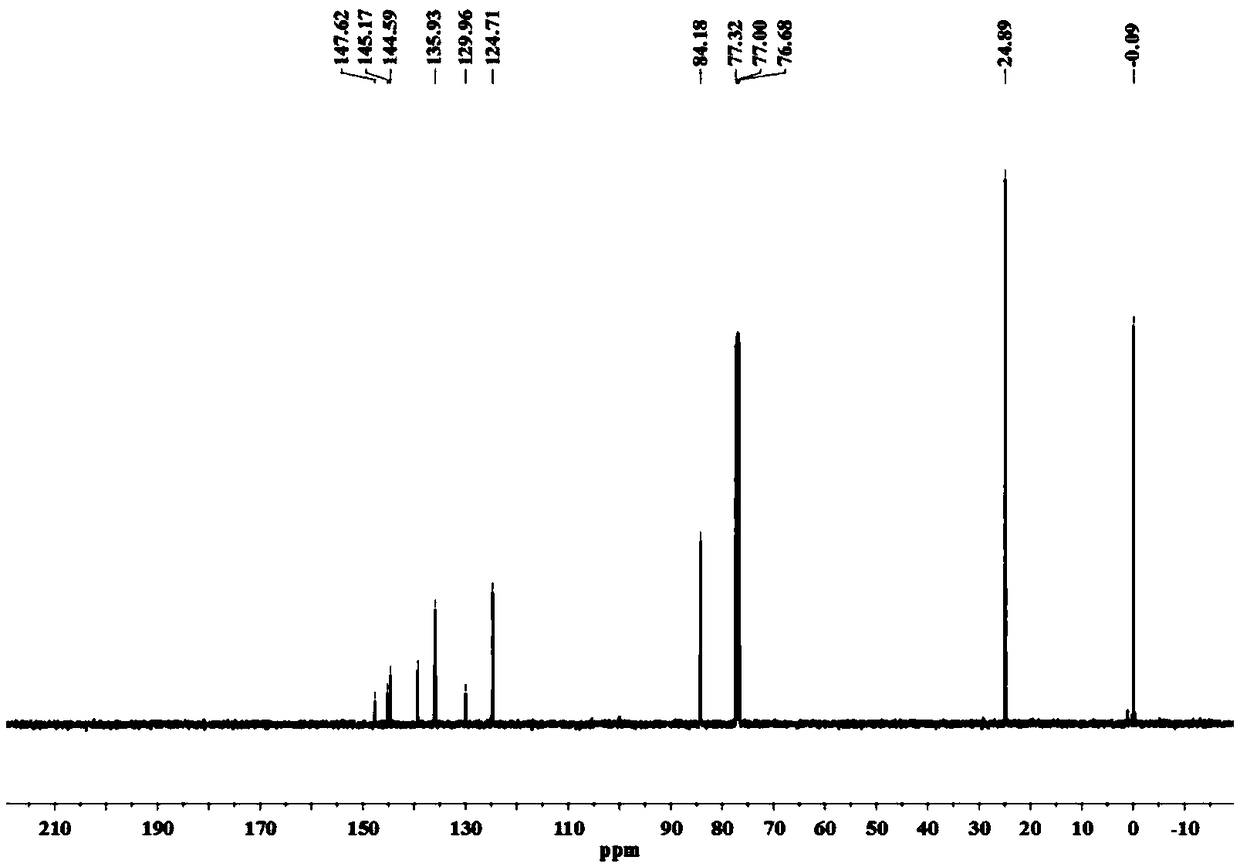
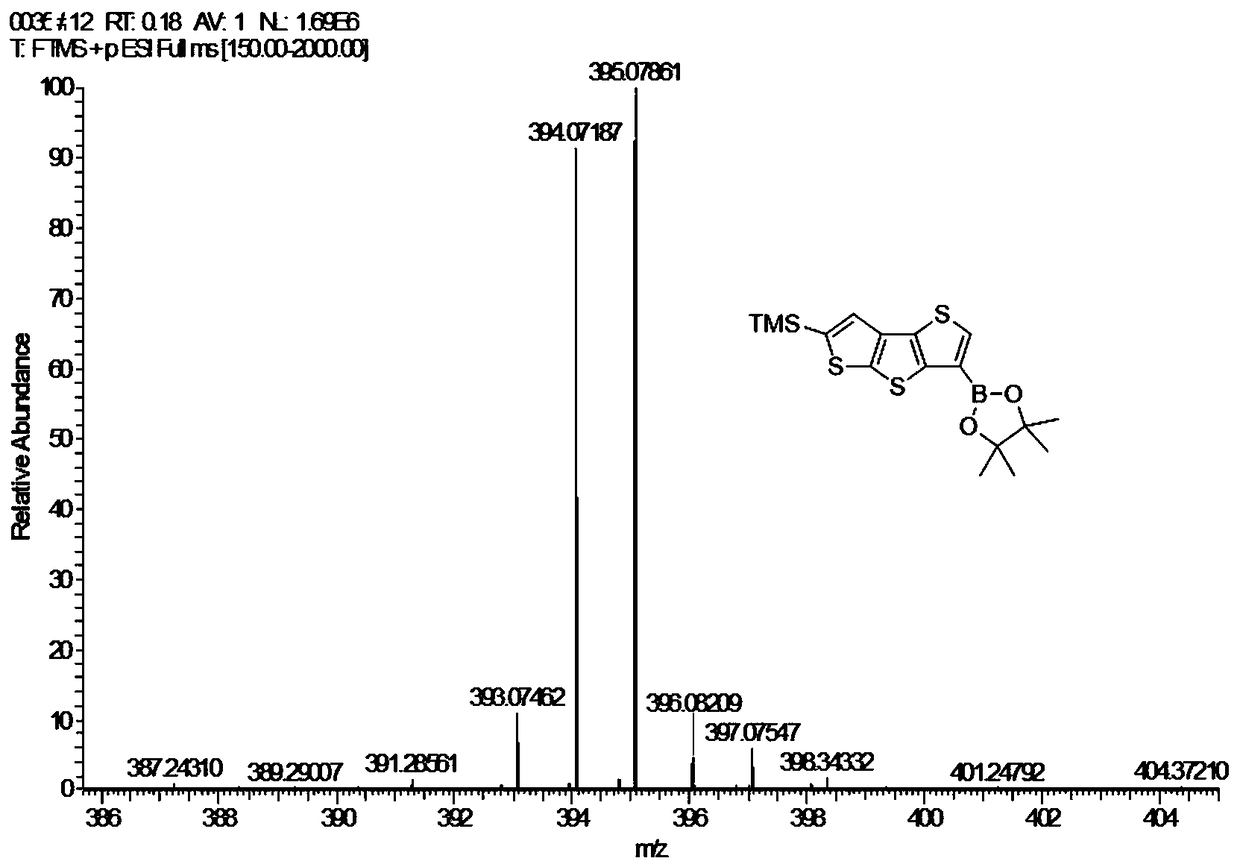
[0050] (1) Preparation of 6-trimethylsilyl-3-pinacol ester-dithieno[2,3-b:2',3'-d]thiophene (3), the reaction equation is as follows:
[0051]
[0052] The specific method is: add compound 2 (679mg, 1.95mmol) to dry 100mL Schlenk, vacuum dry for 0.5h, add 50mL anhydrous ether under the protection of argon, stir well to dissolve, then transfer to -78℃ cryostat Keep stirring under argon for 10min; slowly add t-BuLi (1.28M in Pentane, 3.2mL, 4.10mmol, 2.1eq), react at -78℃ for 2h; add isopropanol pinacol borate (0.47mL , 2.34mmol, 1.2eq), slowly warm to room temperature, and stir for 10h. Add 2mL CH 3 OH quenched the reaction, removed the solvent, and added 30 mL saturated NaHCO 3 Solution, use 4×15mL CH 2 Cl 2 Extract the aqueous phase, combine the organic phases, and use 3×15mL H 2 O wash the organic phase, anhydrous MgSO 4 Fully dry, filte...
Embodiment 2
[0062] This embodiment provides a preparation method of asymmetric heptathiophene substituted with double TMS, including the following steps:
[0063] (1) Preparation of 6-trimethylsilyl-3-pinacol ester-dithieno[2,3-b:2',3'-d]thiophene (3), the reaction equation is as follows:
[0064]
[0065] The specific method is: add compound 2 (678mg, 1.95mmol) to dry 100mL Schlenk, vacuum dry for 0.5h, add 50mL anhydrous ether under the protection of argon, stir well to dissolve, then transfer to -70℃ cryostat Keep stirring for 10min; slowly add n-BuLi (1.28M in Pentane, 3.81mL, 4.88mmol, 2.5eq), and react at -70℃ for 2h; add pinacol diboronate (0.39mL, 1.95mmol) under argon protection , 1eq), slowly warm to room temperature, and stir for 8h. Add 2mLCH 3 OH quenched the reaction, removed the solvent, and added 30 mL saturated NaHCO 3 Solution, use 4×15mL CH 2 Cl 2 Extract the aqueous phase, combine the organic phases, and use 3×15mL H 2 O wash the organic phase, anhydrous MgSO 4 Fully dry, ...
Embodiment 3
[0073] This embodiment provides a preparation method of asymmetric heptathiophene substituted with double TMS, which includes the following steps:
[0074] (1) Preparation of 6-trimethylsilyl-3-pinacol ester-dithieno[2,3-b:2',3'-d]thiophene (3), the reaction equation is as follows:
[0075]
[0076] The specific method is: add compound 2 (679mg, 1.95mmol) to dry 100mL Schlenk, vacuum dry for 0.5h, add 50mL anhydrous ether under the protection of argon, stir well to dissolve, then transfer to -90℃ cryostat Keep stirring in medium for 20min; slowly add t-BuLi(1.28M in Pentane, 3.2mL, 4.10mmol, 2.1eq), react at -90℃ for 5h; add isopropanol pinacol borate (0.59mL , 2.93mmol, 1.5eq), slowly rise to room temperature, and stir for 14h. Add 2mL CH 3 OH quenched the reaction, removed the solvent, and added 30 mL saturated NaHCO 3 Solution, use 4×15mL CH 2 Cl 2 Extract the aqueous phase, combine the organic phases, and use 3×15mL H 2 O wash the organic phase, anhydrous MgSO 4 Fully dry, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com