Three-dimensional melamine sponge complex fe 3+ Methods for Immobilization of Enzymes
A technology of three-dimensional melamine and sponge, applied in the direction of immobilized on or in the inorganic carrier, biochemical equipment and methods, enzymes, etc., can solve the problem of high cost, affecting product quality, free enzyme survival temperature, and narrow pH range and other problems, to achieve the effect of simple recycling and complete three-dimensional structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
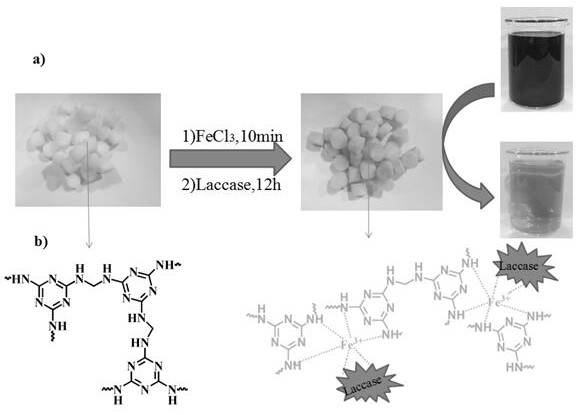
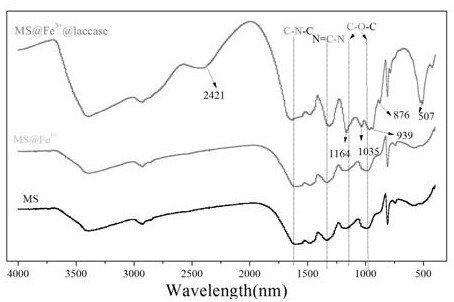
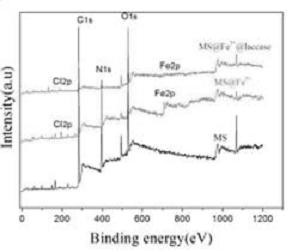
[0025] The melamine sponge purchased in the market is directly punched into a cylindrical shape with a hole diameter of d=1.2cm and a thickness of h=1cm without any treatment. The quality of the sponge is guaranteed to be 10mg (±0.5mg), and the weighed sponge is placed in the 80mM FeCl 3 After soaking in the solution for 10 minutes, take it out with tweezers and squeeze it 5-6 times, blot the water in the sponge with filter paper, and dry it in a vacuum oven at 60°C for 12 hours to obtain the complexed Fe 3+ Materials (MS@Fe 3+ ). Then purify laccase, weigh 300 mg laccase and dissolve in water, centrifuge at 12000 rpm for 15 min in a refrigerated centrifuge at 4°C, take the supernatant to freeze-dry, and store at 4°C for later use. Weigh 100 mg of lyophilized purified laccase and dissolve it in 100 ml, pH=4, 50 mM phosphoric acid buffer, take 20 ml of buffer, add 100 mg of material (MS@Fe 3+ ), and overnight at 25 °C in a shaker. The loading amount of laccase was detected ...
experiment example 1
[0029] Study on Kinetic Equation of Immobilized Enzyme
[0030] By measuring the concentration of different substrates 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) under the condition of 25°C and pH=4 For the reaction rate, calculate the Michaelis-Menten equation parameters of the immobilized enzyme and the free enzyme, including the Michaelis constant Km and the maximum reaction rate Vmax of the immobilized enzyme and the free enzyme. Among them, the Michaelis constant Km represents the affinity between the enzyme and the substrate. The smaller the Km, the stronger the affinity. Vmax represents the maximum reaction rate of the enzyme-catalyzed reaction. The larger the Vmax, the stronger the activity of the enzyme. Such as Figure 4 As shown, the immobilized enzyme and the free enzyme are two straight lines with different slopes. The Vmax and Km values of the immobilized enzyme and the free enzyme are relatively close, and the Vmax of the im...
experiment example 2
[0032] Study on Stability of Immobilized Enzyme
[0033] a.pH stability
[0034] The immobilized enzyme and the free enzyme were respectively placed in the buffer solution of different phosphates with a pH of 2-9, at room temperature, after standing for 12 hours, by measuring 0.5mM substrate 2,2'-azinobis-(3-ethane Oxidation product of diammonium salt ABTS-ABTS +· To determine the activity of free enzyme and immobilized enzyme at different pH. ABTS +· There is a maximum absorption at the ultraviolet wavelength of 415nm. Depend on Figure 5 It can be seen that the immobilized enzyme still retains the characteristics of the free enzyme, and the optimum pH value of it and the free enzyme is around 3, but the immobilized enzyme in MS@Fe 3+ Immobilized enzymes are more resistant to alkaline conditions than free enzymes after the material has been attached.
[0035] b. Thermal stability
[0036] In the environment of pH=4 and temperature 60°C, as the incubation time increases, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com