Methods and compositions comprising tumor suppressor gene therapy and immune checkpoint blockade for the treatment of cancer
An immune checkpoint, cancer technology with applications in biology and medicine that can address issues that have not shown therapeutic benefit, non-specific expression, inefficient delivery, and biosafety, limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
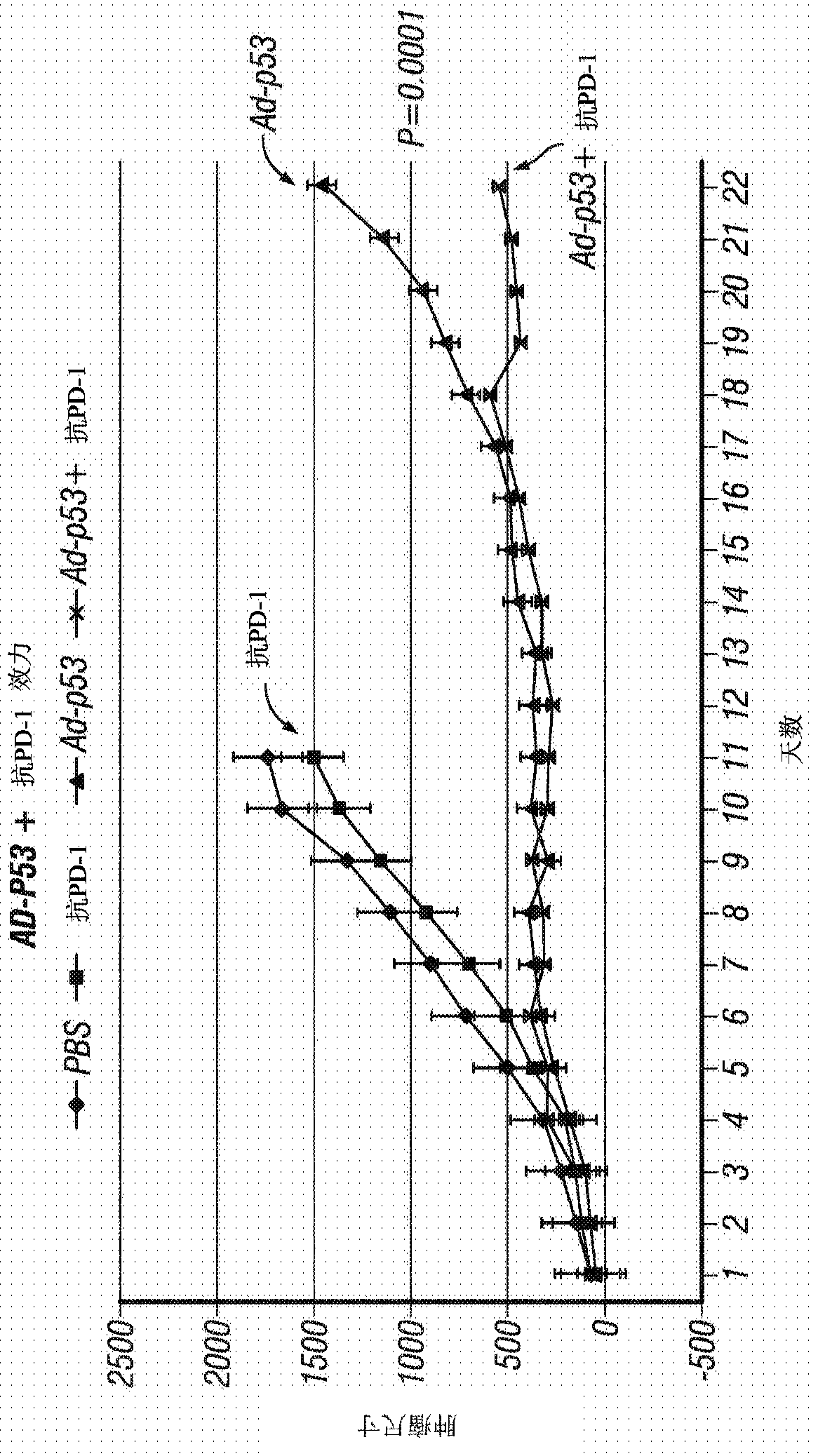
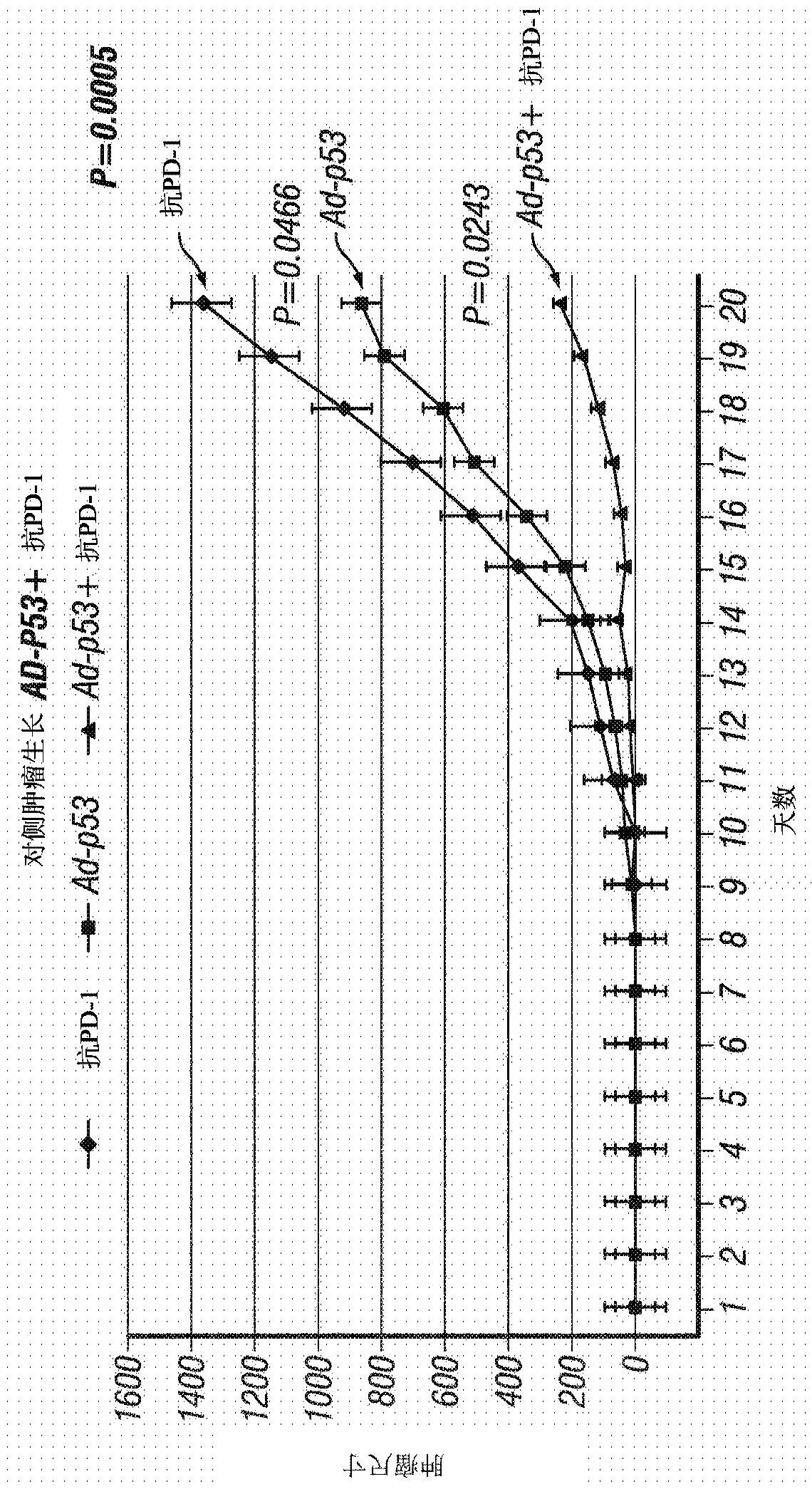
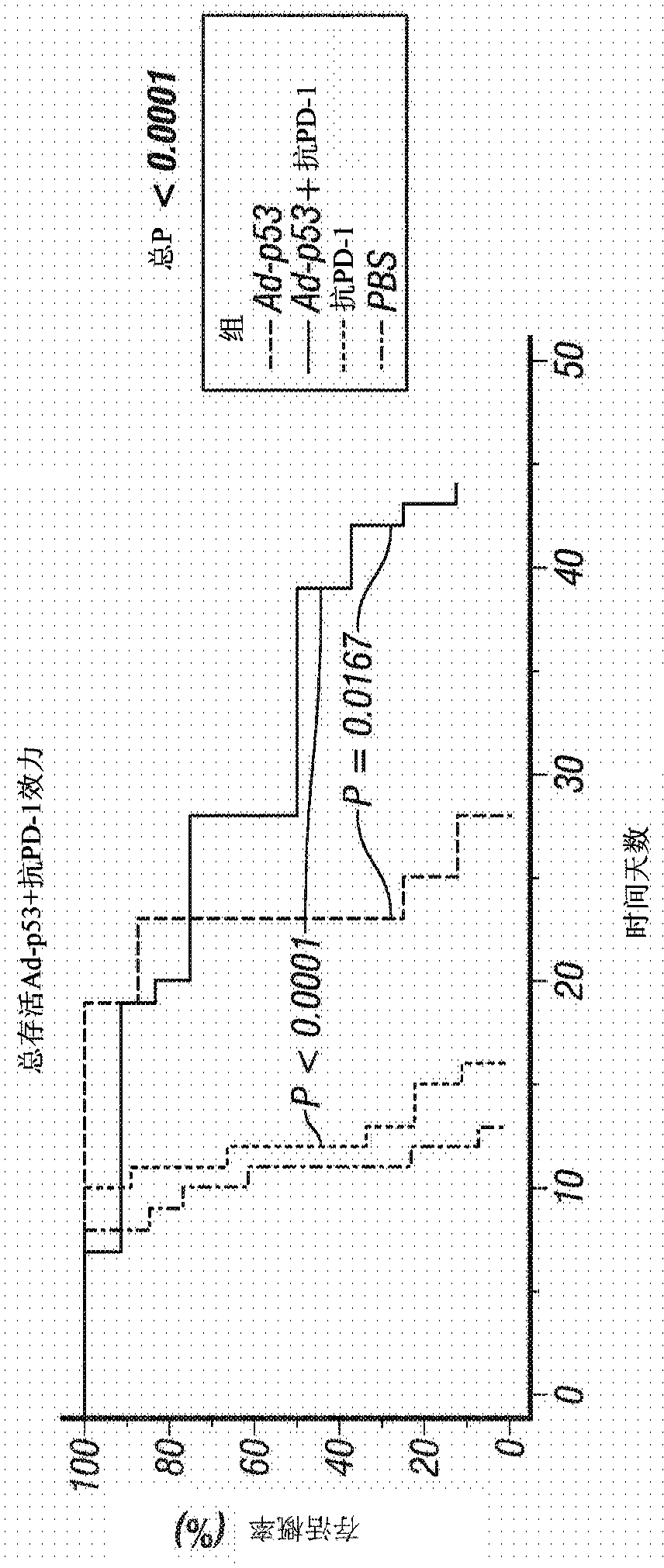
[0256] Example 1 - Ad-p53 or Ad-IL24 Tumor Suppressor Immunogene Therapy for Inducing Distant Effects and Reversing Resistance to Prior Immunotherapy
[0257] The efficacy of tumor suppressor immunogene therapy to induce distant effects in tumors resistant to previous immunotherapy was demonstrated in immunocompetent animal tumor models. Use the following treatments, doses and schedules:
[0258] Animals, Tumor Inoculation and Measurements: C57BL / 6 (B6) mice (6 to 8 weeks old) were used. B16F10 melanoma cells (ATCC, 5 × 10 5 cells / mouse) to form a "primary tumor". When the tumor size reaches about 50mm 3 Start treatment at , and this is referred to as treatment day 1. Tumor growth was monitored by measuring the length (L) and width (w) of the tumor, and the tumor volume was calculated using the following formula: Volume = 0.523L(w) 2 . Animals were monitored for up to 60 days and when tumors reached approximately 2000mm 3 time to execute.
[0259] viral vector : Re...
Embodiment 2
[0266] Example 2 - Combination Ad-p53 and Ad-IL24 Tumor Suppressor Immunogene Therapy for Tumors Resistant to Prior Immunotherapy
[0267] To determine the antitumor effect induced by the combination of tumor suppressors, Ad-p53 and Ad-IL24 were combined and administered as described above, using 50% of the original dose of each vector in the final therapeutic formulation. Such as( Figure 7 ) showed severe tumor progression in animals treated with anti-PD-1 monotherapy, whereas the combination of Ad-p53+Ad-IL24 showed reduced tumor growth. Reversal of anti-PD-1 resistance was observed in animals treated with the combination of Ad-p53+Ad-IL24+anti-PD1, which induced primary tumors, compared to anti-PD1 alone or Ad-p53+Ad-IL24 treatment Maximum reduction in volume. Statistical analysis of variance (ANOVA) comparison of tumor volumes for each treatment confirmed that the combined effect of Ad-p53+Ad-IL24+anti-PD-1 treatment was synergistic at day 14 of treatment (p-value=0.035...
Embodiment 3
[0268] Example 3 - Tumor Suppressor Immunogene Therapy in Combination with Chemotherapy and Cytokine Therapy for Tumors Resistant to Prior Immunotherapy
[0269] Animals, tumor inoculation and measurement, Ad-IL24 vector treatment and antibody treatment were used as described in Example 1.
[0270] chemotherapy and cytokine therapy : Treatment with chemotherapy (5FU and cyclophosphamide CTX) started on day 3 and consisted of a single injection of the drug (5FU and CTX), intraperitoneally (i.p.), using a 1 mL syringe. For 5FU, the dose is 50 mg / kg body weight; for cyclophosphamide, the dose is 80 mg / kg body weight. GM-CSF cytokine treatment was provided as recombinant murine GM-CSF dissolved in sterile ddH2O and adjusted to 1X PBS just prior to use. Animals were treated intraperitoneally and a dose of 0.5 μg / mouse was administered. Treatments were performed twice daily from days 3 to 13 of the study.
[0271] The therapeutic efficacy of 5-FU+CTX+GM-CSF combined with anti-P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com