Gracilaria lemaneiformis antihypertensive peptide extract, gracilaria lemaneiformis antihypertensive peptide and application of gracilaria lemaneiformis antihypertensive peptide extract and gracilaria lemaneiformis antihypertensive peptide
A technology of antihypertensive peptides and extracts, applied in the application fields of antihypertensive peptides, antihypertensive drugs and health care products, to achieve good antihypertensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of asparagus antihypertensive peptide extract comprises the following steps:
[0025] (1) Asparagus powder 20g, add 500ml of distilled water and stir to swell for 2h.
[0026] (2) Add 500mg of trypsin (EC 3.4.23.4, ≥250.N.F.U / mg) for enzymolysis, the enzymolysis temperature is 42°C, the enzymolysis pH is controlled at 8.0, the enzymolysis time is 3h, and the enzyme is extinguished at 100°C after the enzymolysis is completed 10min, filter to remove impurities.
[0027] (3) The supernatant was precipitated with ethanol with a final volume concentration of 70% to remove the precipitate, and the supernatant was ultrafiltered to collect components <3 kDa, concentrated to 20 ml and freeze-dried to obtain the antihypertensive peptide extract.
[0028] (4) Determination of IC50 value of antihypertensive peptide by high performance liquid chromatography.
[0029] The medicines and instruments used in this example can be obtained commercially unless other...
Embodiment 2
[0037] The preparation method of asparagus antihypertensive peptide extract comprises the following steps:
[0038] (1) Asparagus was dried and crushed, 20g of dry powder was added to 400ml of distilled water, stirred, and swelled at room temperature for 4h.
[0039] (2) Add 400mg of trypsin (EC 3.4.23.4, ≥250.N.F.U / mg) and stir for enzymolysis. The enzymolysis temperature is controlled at 42°C, the enzymolysis pH is controlled at 7.8, and the enzymolysis time is 2 hours. After the enzymolysis is completed, boil water Inactivate the enzyme for 10 min, filter to remove impurities.
[0040] (3) The supernatant is precipitated with ethanol with a final volume concentration of 80% to remove the precipitate, and the supernatant is ultrafiltered to collect <3kDa components, and concentrated to 20ml, and freeze-dried to obtain the Asparagus antihypertensive peptide extract .
[0041](4) Evaluate the ACE inhibitory activity of the Asparagus antihypertensive peptide extract by using ...
Embodiment 3
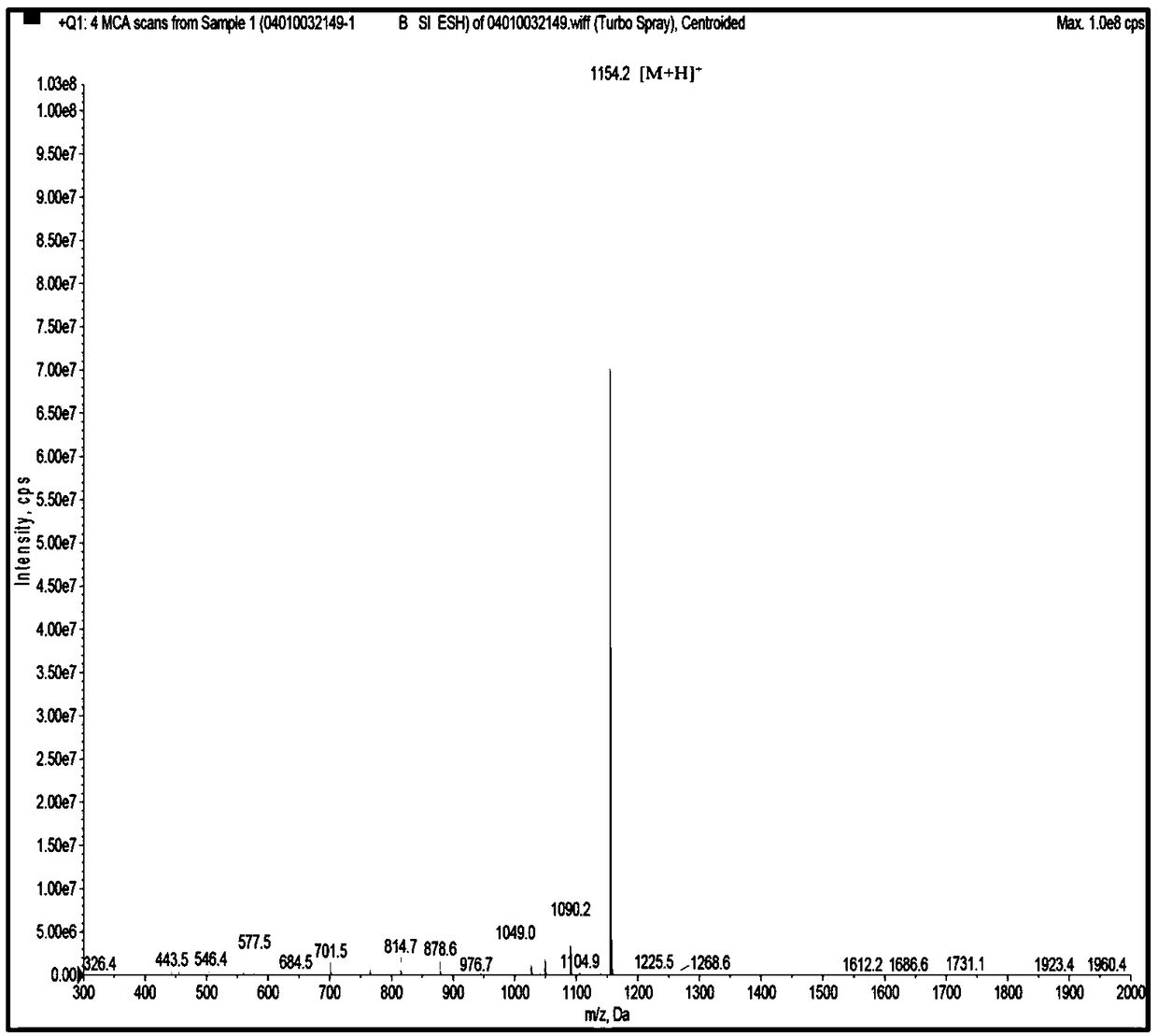
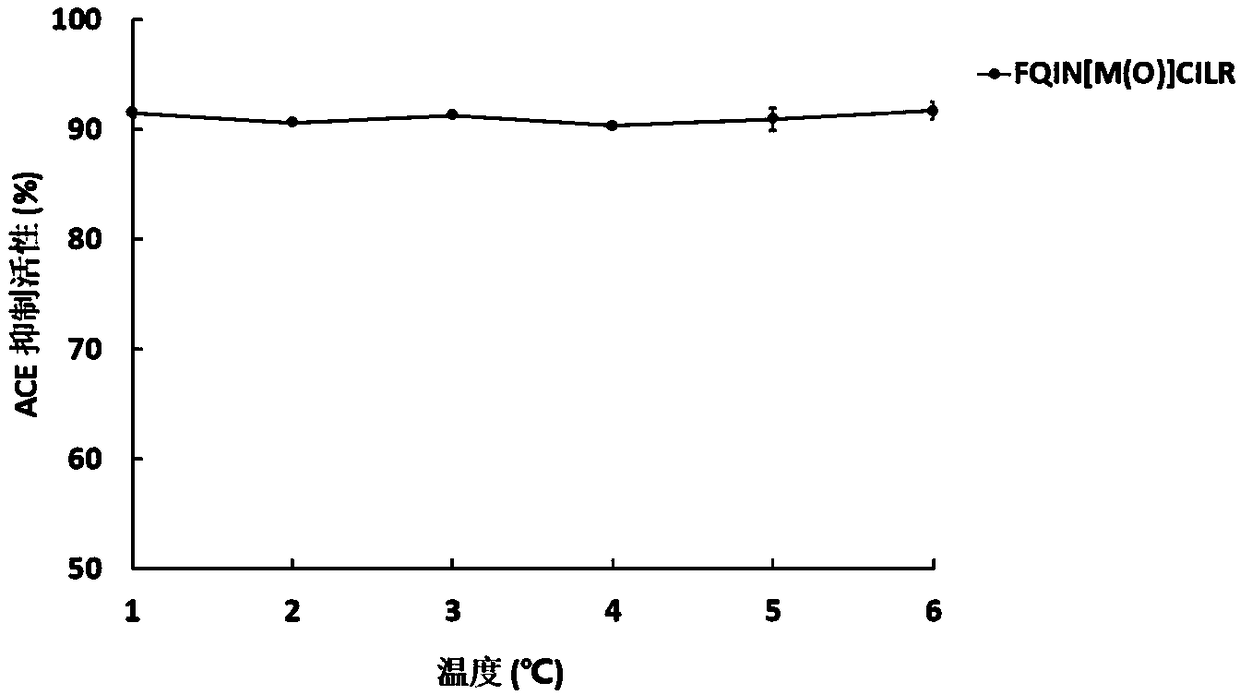
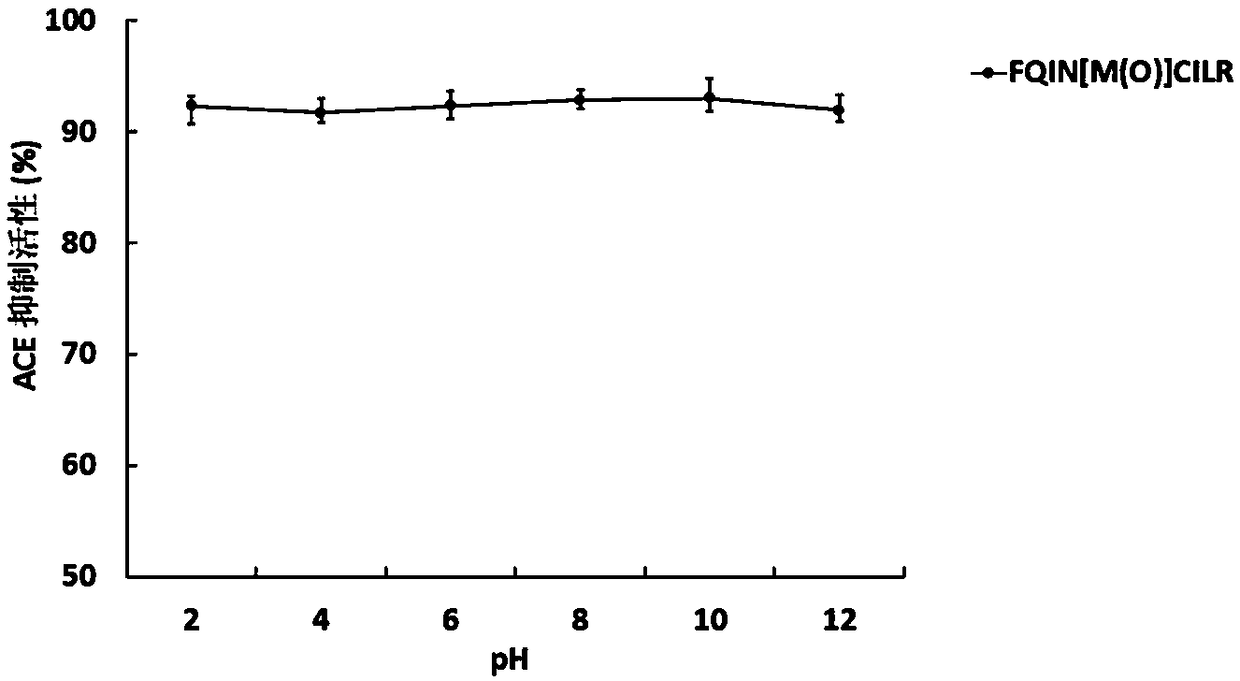
[0046] The UPLC-ESI-Q-Exactive Focus-MS / MS mass spectrometry information of the above-mentioned asparagus <3kDa antihypertensive peptide extract of asparagus was used Mascot2.2 and asparagus transcriptome (Accession: SRX258772, downloaded from NCBI) database For comparison, the top 20 polypeptides were selected for selective synthesis, and then the polypeptides with ACE inhibitory activity were screened. It was identified that the polypeptide FQINMCILR had better ACE inhibitory activity. Methionine and cysteine are easily oxidized in the body because they contain S atoms, and the S atoms are modified into a sulfoxide structure to enhance their stability in the body. FQINMCILR commissioned Shanghai Qiangyao Biotechnology Co., Ltd. to synthesize and selectively modify methionine to obtain a peptide with the sequence FQIN[M(O)]CILR, a purity of ≥98%, and a molecular weight of 1153.43Da identified by mass spectrometry.
[0047] (1) Determination of ACE inhibitory activity of pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com