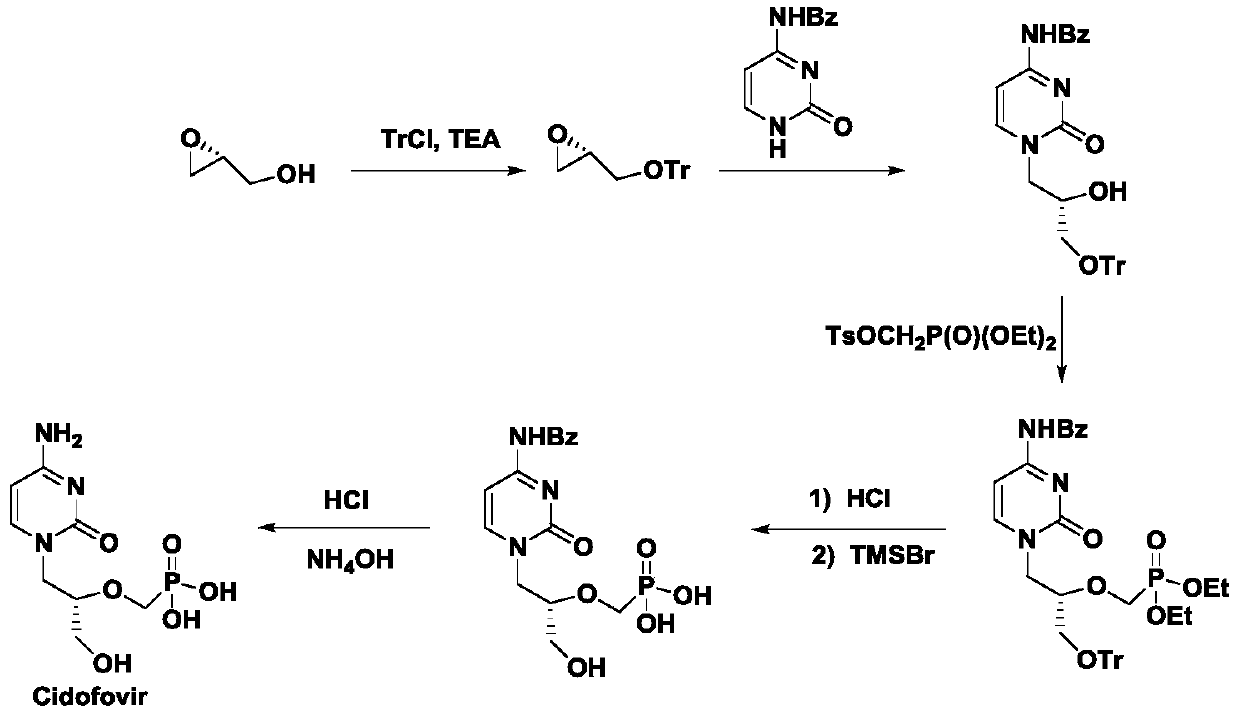
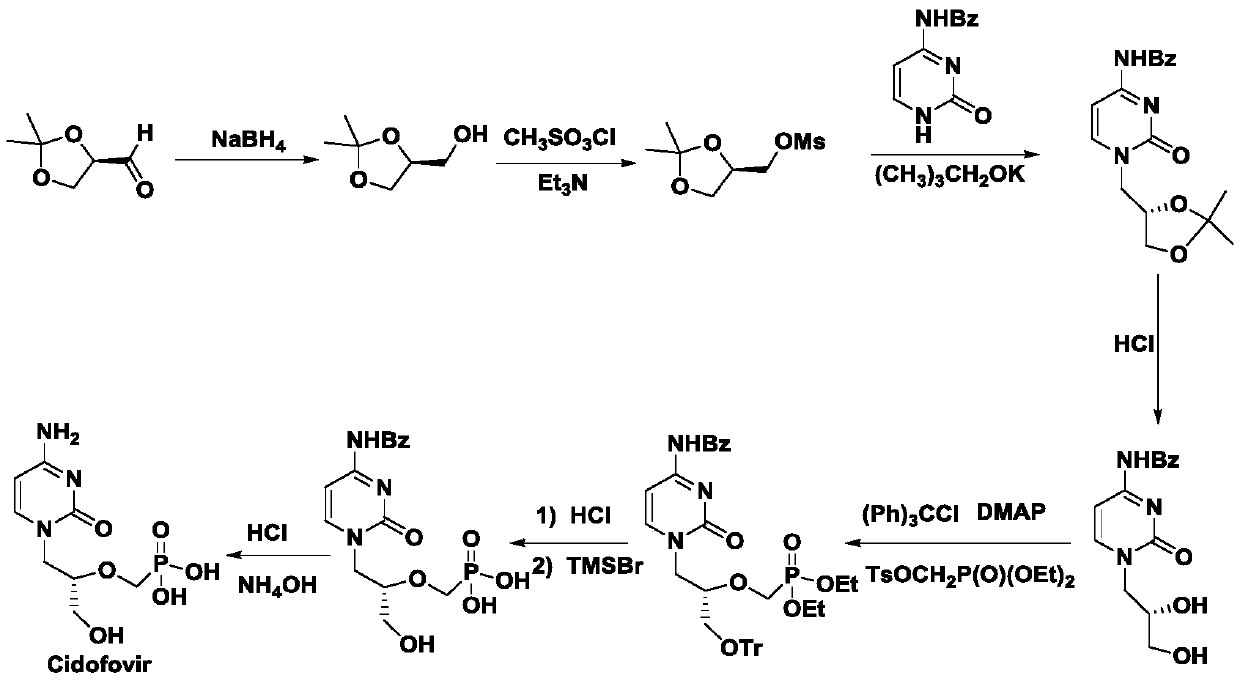
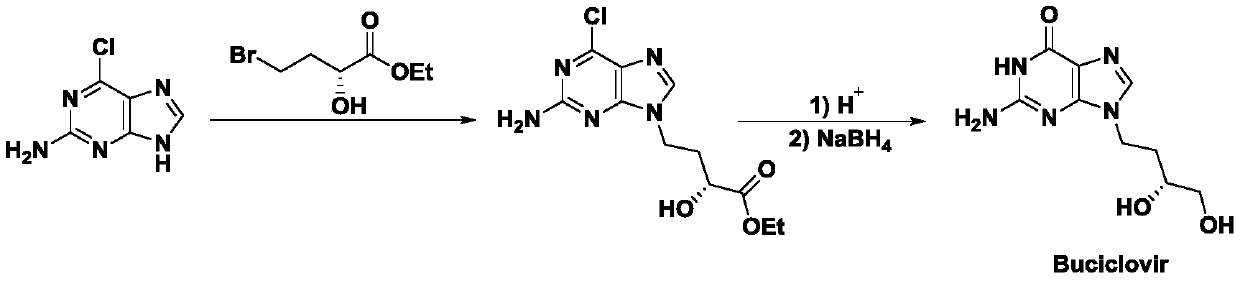
A method for synthesizing antiviral drug cidofovir intermediates and buciclovir intermediates
A technology of antiviral drugs and intermediates, applied in the field of synthetic antiviral drugs, can solve the problems of cumbersome and complicated process, high cost, and difficult preparation of chiral intermediates, and achieve the effect of high stereoselectivity and easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031]
[0032] a Reaction conditions: the reactions were carried out on a 1a(0.05mmol),K 2 CO 3 (6equiv, 0.3mmol, 41.4mg), K 3 Fe(CN) 6 (6equiv, 0.3mmol, 98.7mg) and 10mol% L * . b Yield of isolated product. c Determined by chiral HPLC analysis. d Reaction temperature, at 0℃,
[0033] During the screening of reaction conditions, the effects of different chiral quinine ligands and temperature on the reaction were investigated. finalized L 2 The optimal ligand reaction temperature is 0°C.
[0034] Investigation of reaction conditions:
[0035] In the reaction bottle, the (DHQD) 2 PHAL (3.9mg, 0.005mmol), K 3 Fe(CN) 6 (49.4mg, 0.15mmol), K 2 CO 3 (20.7mg, 0.15mmol), K 2 OSo 2 (OH) 4 (0.36mg, 0.001mmol), MeSO 2 NH 2 (9.5 mg, 0.1 mmol) and a mixed solvent of water and tert-butanol (volume ratio 1 / 2, the same below, 2 mL) were added sequentially, and stirred at room temperature for 30 minutes. The olefin was added to the reaction mixture, and the...
Embodiment 2
[0046]
[0047] In the reaction bottle, sequentially add (DHQD) 2 PHAL (3.9mg, 0.005mmol), K 3 Fe(CN) 6 (49.4mg, 0.15mmol), K 2 CO 3 (20.7mg, 0.15mmol), K 2 OSo 2 (OH) 4 (0.36mg, 0.001mmol), MeSO 2 NH 2 (9.5mg, 0.1mmol) and a mixed solvent of water and tert-butanol (1 / 2, 2mL). The mixture was stirred at room temperature for 30 minutes. The 2'-trimethylsilyl substituted 4-amino benzoyl protected cytosine was then added to the reaction mixture, and the heterogeneous slurry was stirred at 0°C for 12 hours, monitored by TLC. By adding Na 2 S 2 o 3 The reaction was quenched at 0 °C, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was then partitioned between ethyl acetate and water. The combined organic phase Na 2 SO 4 Dry, filter and concentrate in vacuo to give a crude oil. Then obtain white solid through column chromatography, yield 91%, 95%ee; Characterization data [α] 25 D =33.5(c=1.4, MeOD); ee value detection method: HP...
Embodiment 3
[0049]
[0050] In the reaction bottle, sequentially add (DHQD) 2 AQN (4.3mg, 0.005mmol), K 3 Fe(CN) 6 (49.4mg, 0.15mmol), K 2 CO 3 (20.7mg, 0.15mmol), K 2 OSo 2 (OH) 4 (0.36mg, 0.001mmol), MeSO 2 NH 2 (9.5mg, 0.1mmol) and a mixed solvent of water and tert-butanol (1 / 2, 2mL). The mixture was stirred at room temperature for 30 minutes. The 3'-triethylsilyl substituted purine was then added to the reaction mixture, and the heterogeneous slurry was stirred at 0°C for 12 hours, monitored by TLC. By adding Na 2 S 2 o 3 The reaction was quenched at 0 °C, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was then partitioned between ethyl acetate and water. The combined organic phase Na 2 SO 4 Dry, filter and concentrate in vacuo to give a crude oil. Then obtain white solid through column chromatography, yield 96%, 95%ee; Characterization data [α] 25 D =43.17(c=0.99, MeOH); ee value detection method: HPLC (IE, n-hexane / 2-propanol=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com