C10+ heavy aromatic hydrocarbon selective hydrogenation ring-opening catalyst and preparation method thereof
A hydrogenation ring-opening and catalyst technology, which is applied in the direction of molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of ineffective utilization and achieve reasonable distribution of acidity and precious metals, high activity, and improved synergistic effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
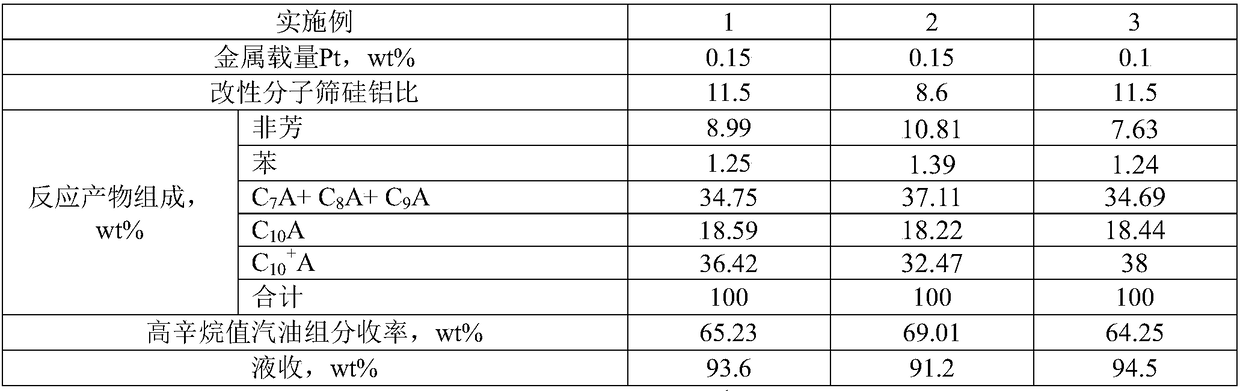
Embodiment 1
[0028] (1) Dissolve 40 g of ammonium fluorosilicate in 1000 mL of deionized water to make ammonium fluorosilicate solution;
[0029] (2) Dissolve 5g of chloroplatinic acid hexahydrate in 500mL of deionized water to obtain a chloroplatinic acid solution;
[0030](3) Add 100g HY molecular sieve into 600mL deionized water and stir at 90°C, and add 1000mL of ammonium fluorosilicate solution dropwise at a rate of 5mL / min, and add 1 / 2 of the ammonium fluorosilicate solution dropwise. Add the same amount of chloroplatinic acid solution when the total amount is added dropwise to the ammonium fluorosilicate solution, and the total amount of Pt added is 0.1% of the final catalyst weight; the ammonium fluorosilicate solution and the chloroplatinic acid solution are all added dropwise After that, keep stirring and aging for 3h;
[0031] (4) After aging, filter and wash with deionized water until no fluorine element can be detected in the washing liquid, dry at 120°C for 8h, and roast at ...
Embodiment 2
[0038] (1) Dissolve 40 g of ammonium fluorosilicate in 1000 mL of deionized water to make ammonium fluorosilicate solution;
[0039] (2) Dissolve 5g of chloroplatinic acid hexahydrate in 500mL of deionized water to obtain a chloroplatinic acid solution;
[0040] (3) Add 100g HY molecular sieve into 600mL deionized water and stir at 90°C, and add 1000mL of ammonium fluorosilicate solution dropwise at a rate of 10mL / min, and add 1 / 2 of the ammonium fluorosilicate solution dropwise. The same amount of chloroplatinic acid solution was added when all the ammonium fluorosilicate solution was added dropwise, and the total amount of Pt added was 0.1% of the final catalyst weight. After the ammonium fluorosilicate solution and the chloroplatinic acid solution are all added dropwise, continue to stir and age for 3 hours;
[0041] (4) After aging, filter and wash with deionized water until no fluorine element can be detected in the washing liquid, dry at 120°C for 8h, and roast at 500°C...
Embodiment 3
[0046] (1) Dissolve 40 g of ammonium fluorosilicate in 1000 mL of deionized water to make ammonium fluorosilicate solution;
[0047] (2) Dissolve 5g of chloroplatinic acid hexahydrate in 500mL of deionized water to obtain a chloroplatinic acid solution;
[0048] (3) Add 100g HY molecular sieve into 600mL deionized water and stir at 90°C, and add 1000mL of ammonium fluorosilicate solution dropwise at a rate of 5mL / min, and add 1 / 2 of the ammonium fluorosilicate solution dropwise. The same amount of chloroplatinic acid solution was added when all the ammonium fluorosilicate solution was added dropwise, and the total amount of Pt added was 0.05% of the final catalyst weight. After the ammonium fluorosilicate solution and the chloroplatinic acid solution are all added dropwise, continue to stir and age for 3 hours;
[0049] (4) After aging, filter and wash with deionized water until no fluorine element can be detected in the washing liquid, dry at 120°C for 8h, and roast at 500°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com