Serum small dense low-density lipoprotein cholesterol detection kit
A low-density lipoprotein and detection kit technology, which is used in the determination/inspection of microorganisms, biochemical equipment and methods, etc., to achieve the effects of good stability, guaranteed accuracy and simple use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
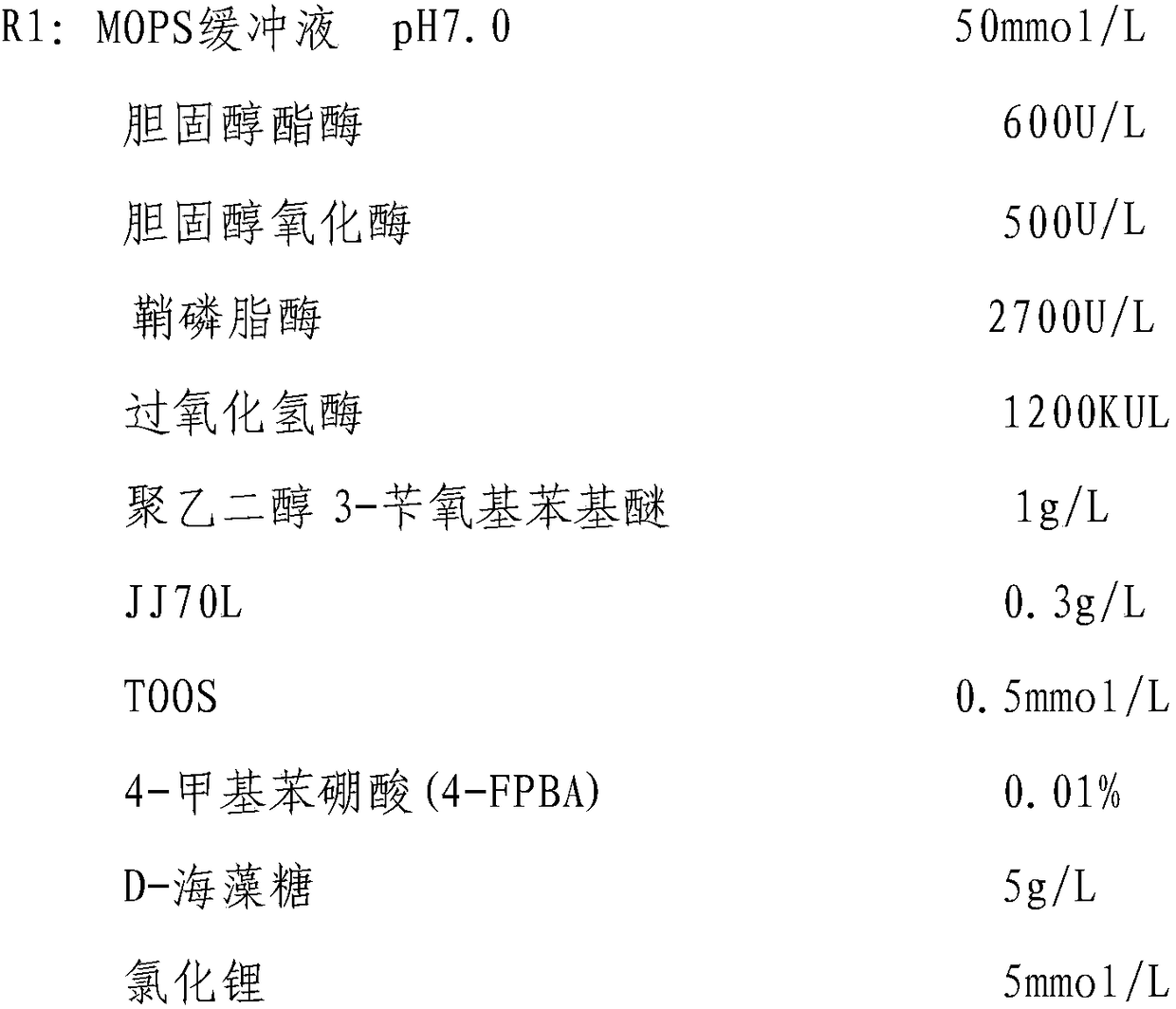
[0040]
[0041] R2:
[0042]
[0043] (2) The usage method of the reagent of this embodiment:
[0044] The serum small and dense low-density lipoprotein cholesterol (peroxidase method) detection reagent described in this embodiment adopts an automatic biochemical analyzer during use, such as Hitachi 7180 automatic analyzer, etc., and utilizes the endpoint method for determination. Set the ratio of sample and reagent R1, R2 to 3:300:100, the detection wavelength is 600nm, place distilled water, standard and sample in the corresponding position of the sample plate, the operation is shown in Table 1:
[0045] Table 1 Example 1 reagent detection method
[0046]
[0047] Serum small dense low-density lipoprotein cholesterol content (mmol / L)=(sample ΔA) / (standard ΔA)×standard concentration.
Embodiment 2
[0049] Interference test: take fresh mixed serum, divide it into 2 equal parts, then divide each equal part into 5 equal parts, add different interfering substances, so that the concentration in the serum reaches the requirements in Table 2. Then respectively use the reagent obtained in Example 1 to compare and measure the content of serum small and dense low-density lipoprotein cholesterol simultaneously with the common and recognized serum small and dense low-density lipoprotein cholesterol reagents in the market. The measurement results of each group are shown in Table 2. Relative deviation (%) = (measuring mean value of interference samples - measuring mean value of control samples) / measuring mean value of control samples × 100%.
[0050] Table 2 embodiment reagent anti-interference performance comparison
[0051]
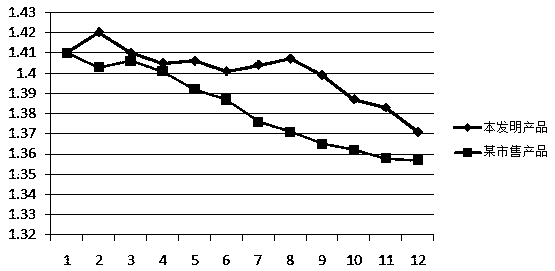
[0052]As can be seen from Table 2, the reagent of embodiment 1 has no effect on the test results when bilirubin≤300 μmol / L, creatinine≤200 μmol / L, hemogl...
Embodiment 3
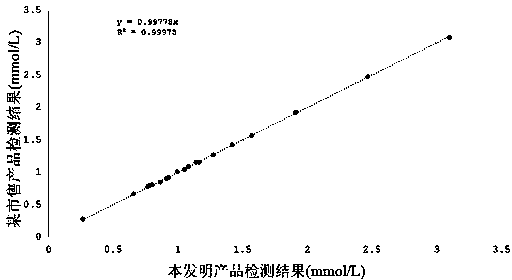
[0054] Correlation experiment: Utilize the formula preparation reagent of embodiment 1, carry out control detection with the small and dense low-density lipoprotein cholesterol (peroxidase method) kit of a certain company approved by the State Food and Drug Administration common in the market, detect simultaneously Twenty clinical serum samples were collected, and the test results are shown in Table 3. And obtained the correlation curve of the two reagents (such as figure 1 As shown), the test results show that the correlation coefficient of the two kits is 0.999, which shows that there is a great correlation between the two.
[0055] Table 3 embodiment 1 reagent and control group kit comparative detection result
[0056]
[0057] The calibrators and quality controls used in the test were:
[0058] Calibrator: the content of serum small dense low-density lipoprotein cholesterol is 1.43mmol / L.
[0059] Quality Control: The target value of serum small dense LDL cholesterol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com