Serum/Plasma Peptide Markers Related to Aided Early Diagnosis of Gestational Diabetes Mellitus and Its Application
An early diagnosis and marker technology, applied in the field of proteomics, to achieve the effect of convenient and easy early diagnosis, rapid and accurate grasp of the patient's condition, and reduction of adverse pregnancy outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
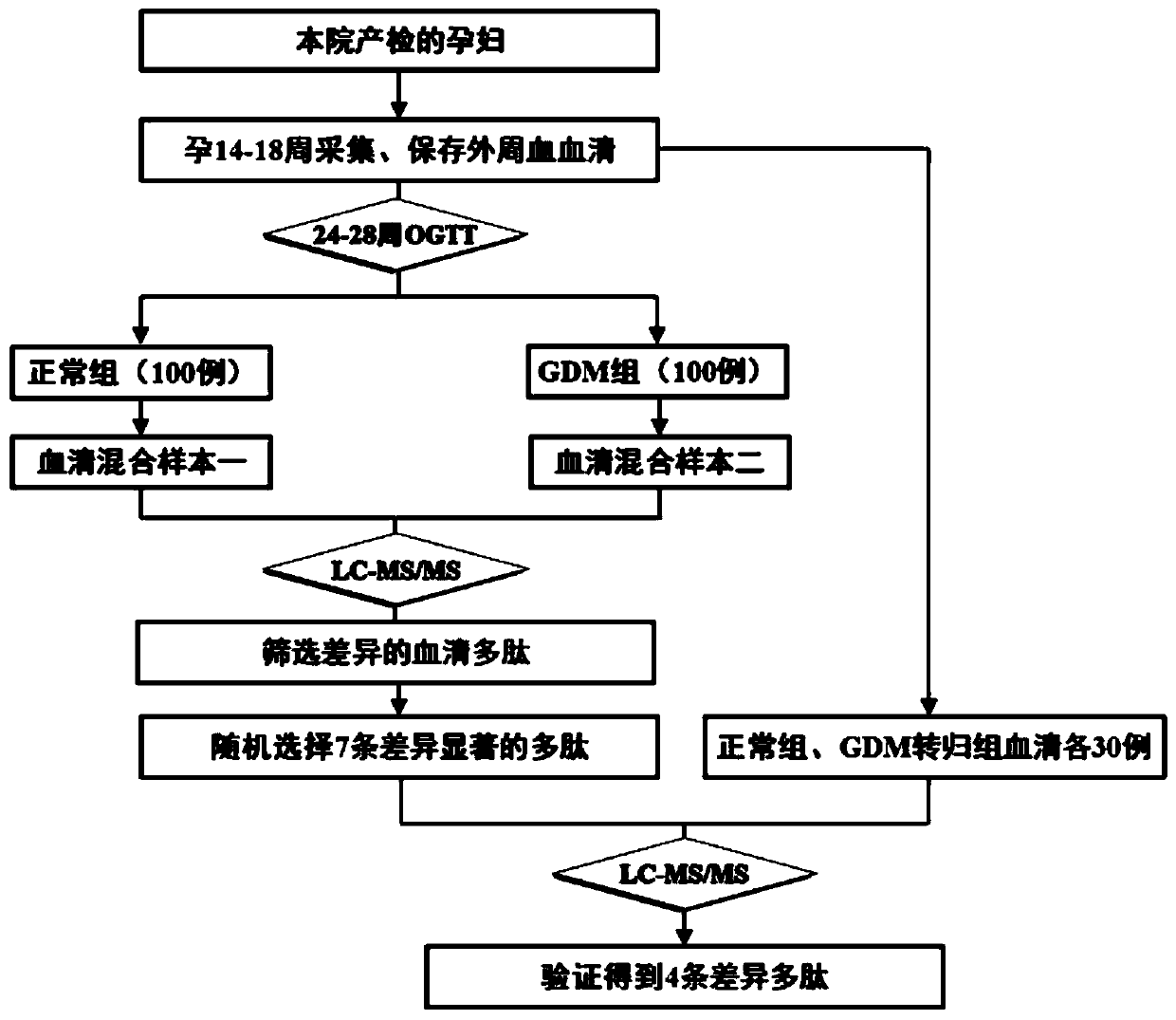
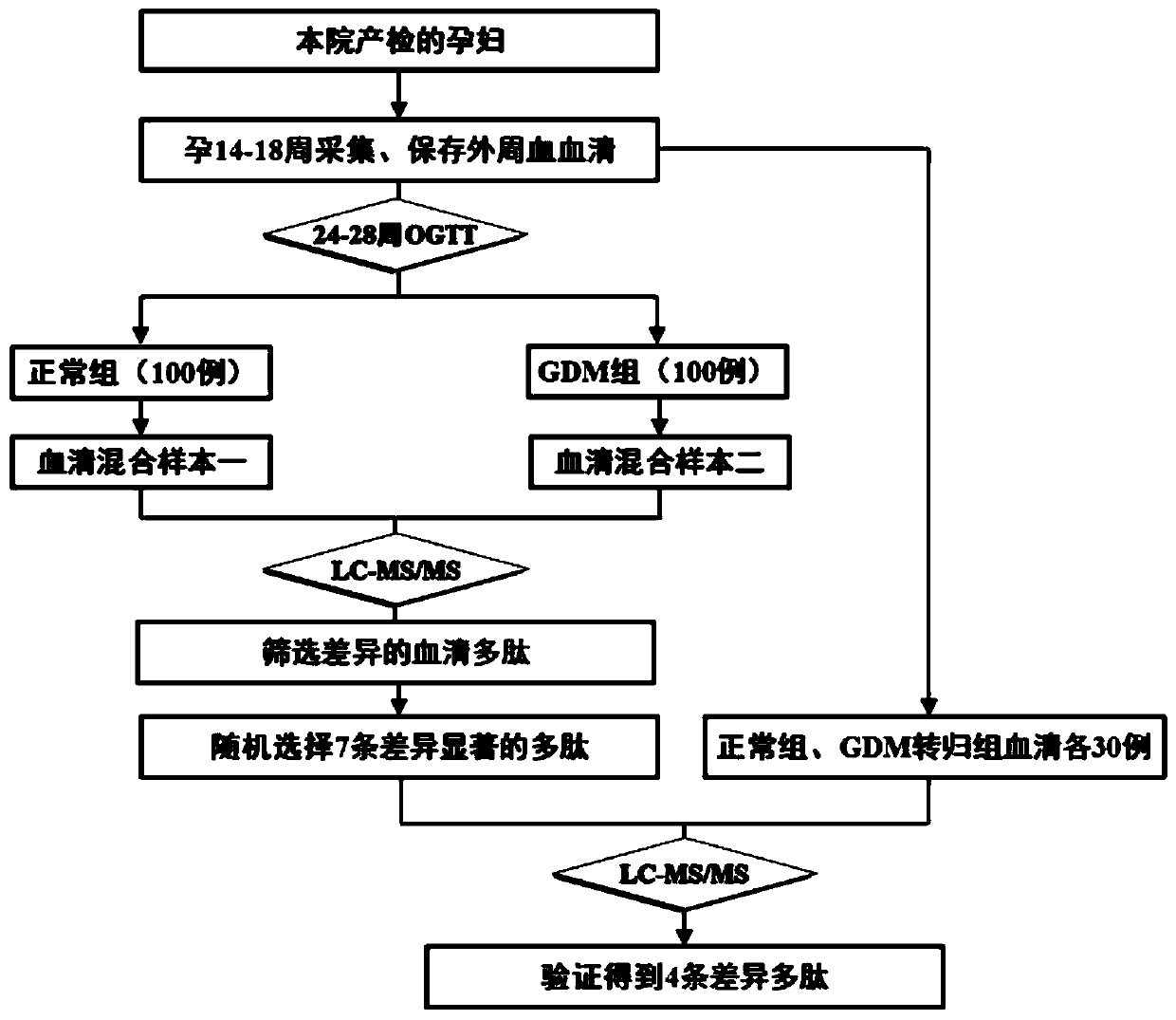
[0025] Embodiment 1, the collection of sample and the arrangement of sample data
[0026] The inventor collected a large number of peripheral blood samples from pregnant women at 14-18 weeks of pregnancy from Nanjing Maternal and Child Health Hospital from April to August 2015 (the samples used for research were collected at the same period, and the sampling, packaging, and storage conditions were uniform). By sorting out the sample data, the inventor selected 200 samples that meet the following criteria as experimental samples:
[0027] 1. Healthy pregnant women at 14-18 weeks of gestation at the time of blood collection
[0028] 2. Pregnant women who were diagnosed as GDM by OGTT during GDM screening at 24-28 weeks of gestation were defined as cases
[0029] 3. The above-mentioned research subjects did not develop GDM during GDM screening at 24-28 weeks of gestation. Healthy pregnant women who matched the age, BMI, and gestational weeks of the cases were defined as controls...
Embodiment 2
[0030] Embodiment 2, sample preparation, proteomics (peptidomics) screening and bioinformatics analysis
[0031] The above 100 eligible GDM cases and 100 healthy controls were subjected to ultrafiltration, TMT, LC-MS / MS and other experiments. The specific steps are as follows:
[0032] Collection preparation and ultrafiltration of samples in serum / plasma
[0033] 1. Centrifuge the sample at 3,000 rpm for 15 minutes at 4°C, and store the upper layer at -20°C for further analysis. Usually 200-250ul serum or plasma can be obtained.
[0034] 2. Centrifuge the sample at 12,000 revolutions per minute at 4°C for 15 minutes, add acrylcyanide to the centrifuged supernatant, then vortex briefly, place it at room temperature for 20 minutes, and then use it according to the instructions, using 10kDa MWCO Peptides below 10 kDa were obtained by filter (Millipore, USA) and lyophilized.
[0035] 3. The protein concentration was measured by using BCA (Pierce, Rockford, IL, USA).
[0036] 4...
Embodiment 3
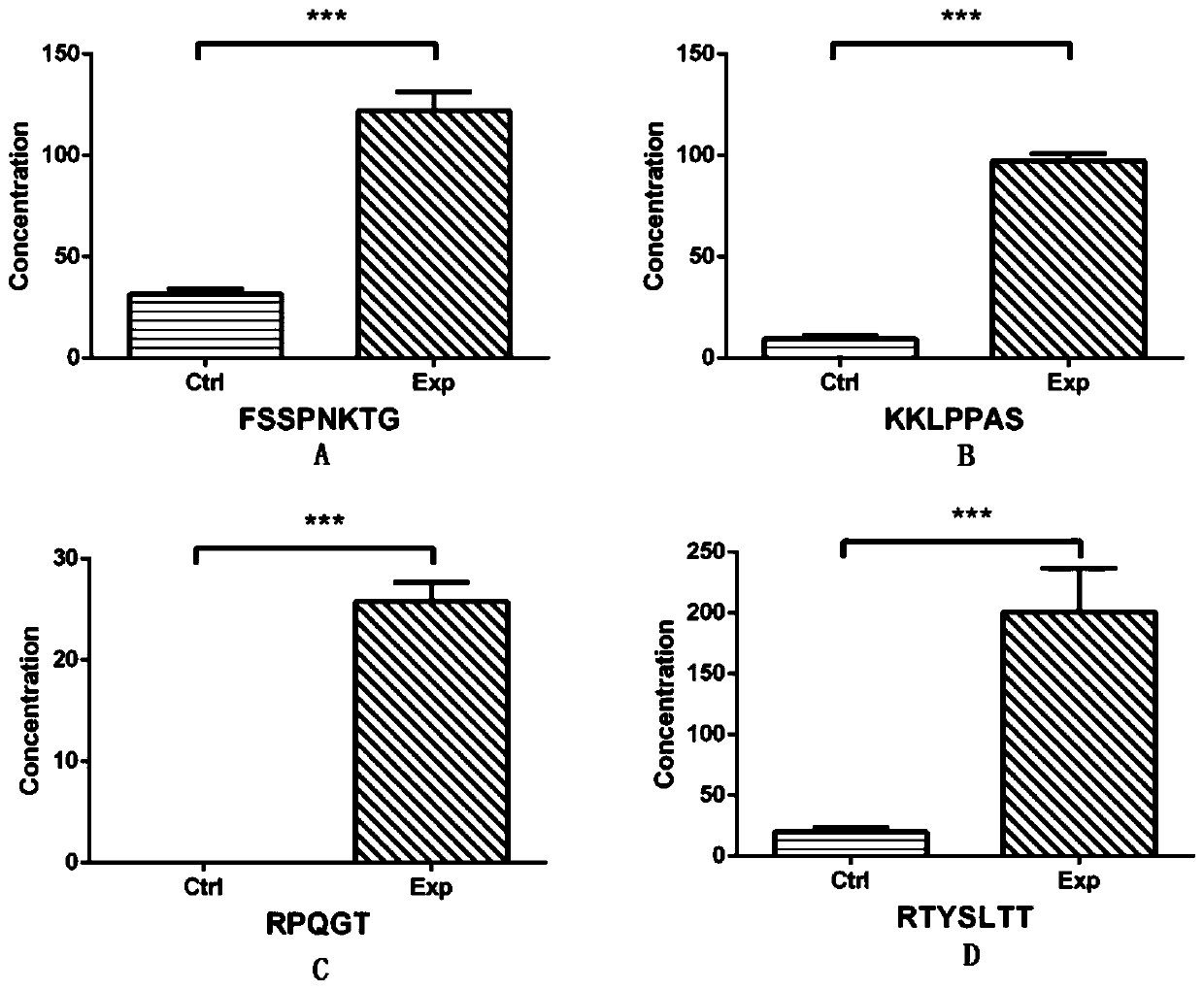
[0060] Embodiment 3, random verification
[0061] According to the above results, 7 differential polypeptides that were significantly highly expressed in the GDM outcome group or only expressed in the GDM outcome group were randomly selected as verification targets. In our early established serum bank at 14-18 weeks of pregnancy, the normal outcome group was selected. and 30 serum samples from the GDM outcome group (the two groups matched each other in terms of age, BMI, and gestational age), further verifying the results of our preliminary screening, and the study confirmed that 4 of the 7 peptides (Q6PFW1, P35658, P32004, and Q9Y4H2 ) expression patterns are basically consistent with the results of primary screening (Table 2), and there are significant differences in the expression between the two groups, suggesting that they are associated with the onset of gestational diabetes mellitus, and they may become molecular markers for early prediction and diagnosis of GDM ( figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com