Parecoxib sodium long-acting preparation and preparation method thereof
A parecoxib sodium, long-acting technology, applied in the field of parecoxib sodium long-acting preparations and its preparation, can solve the problems of excessive appearance, shrinkage, unqualified products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029]
Embodiment 2
[0031]
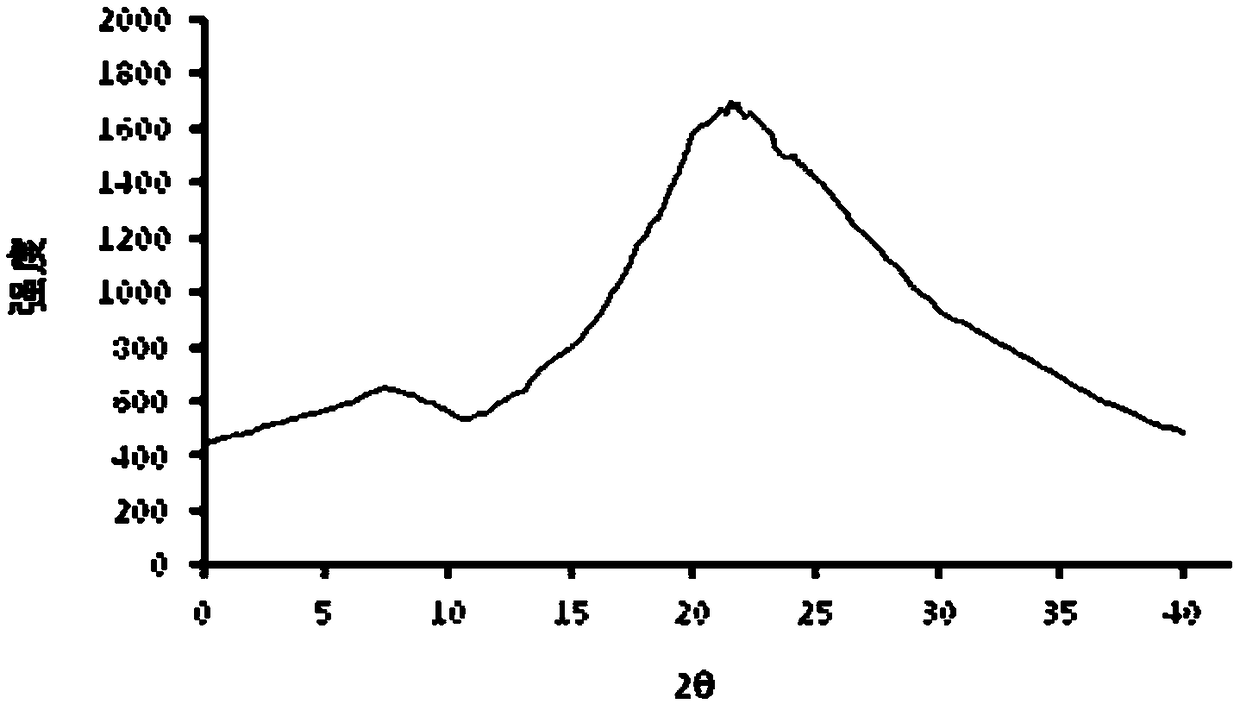
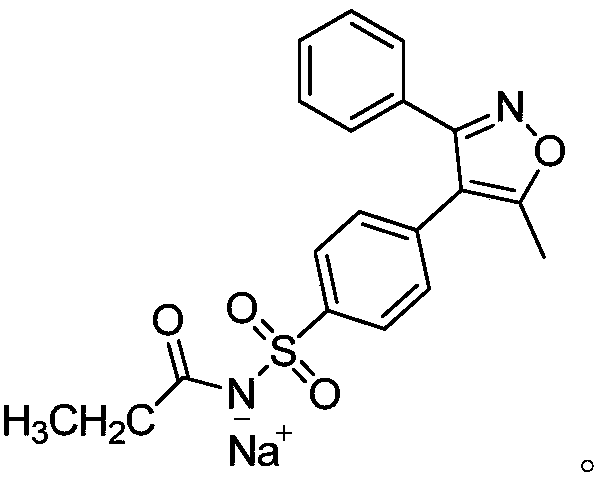
[0032] The parecoxib sodium is in an invisible state, and the X-ray diffraction pattern is as follows: figure 1 shown.
[0033] The preparation method of the amorphous state is as follows: add 1g of parecoxib sodium to 15mL of methanol and heat at 70°C, add 5mL of ethyl acetate under temperature control at 70°C, stir for 10min, slowly cool to 5°C, and crystallize to obtain the amorphous state state of parecoxib sodium.
[0034] The preparation method is as follows:
[0035] Mix parecoxib sodium, croscarmellose sodium, karaya gum and polyvinyl butyral with surfactants and freeze-drying excipients evenly, and freeze-dry to obtain the ready-made preparation.
Embodiment 3
[0037]
[0038]
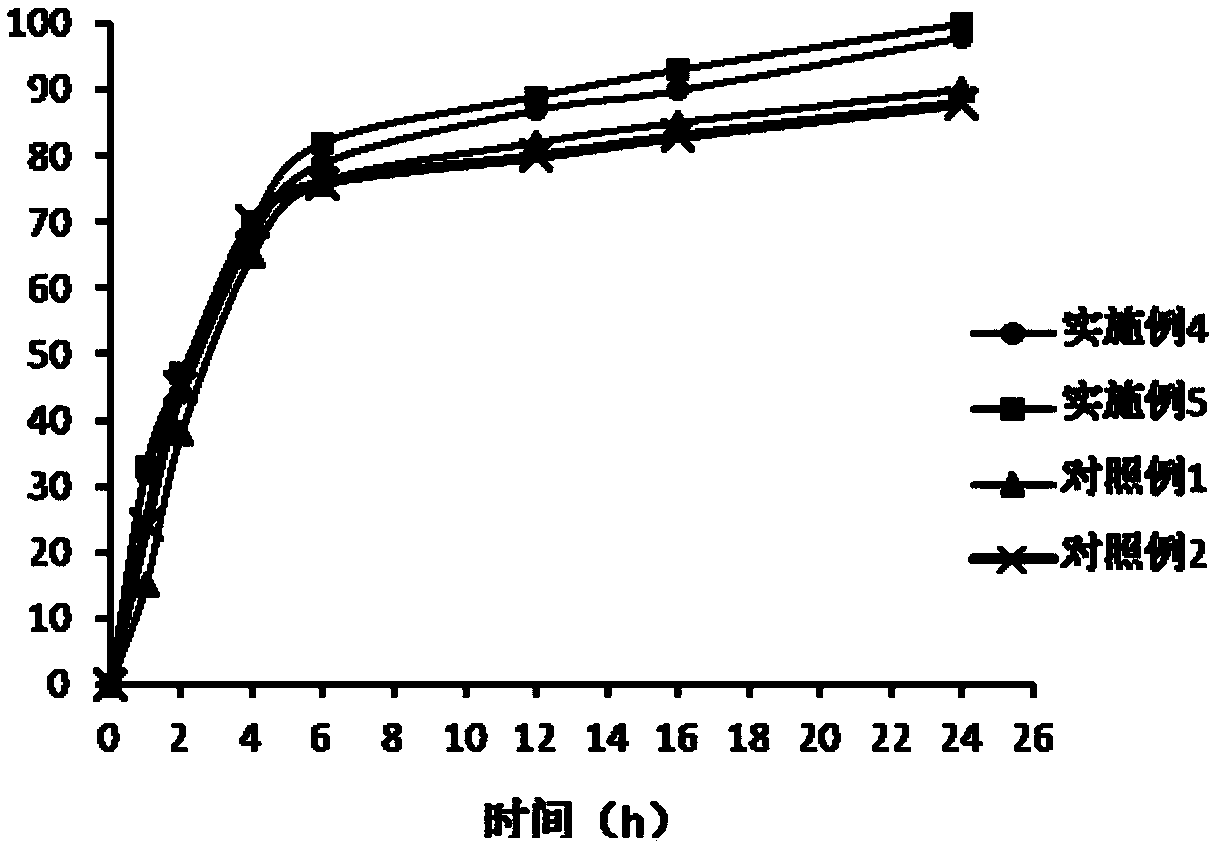
[0039] The parecoxib sodium, cross-linked carboxymethyl cellulose sodium, karaya gum and polyvinyl butyral form microspheres, and the microspheres are prepared by the following method: cross-linked carboxymethyl fiber Sodium sodium is dispersed in twice the amount of water, and stirred evenly to obtain a dispersed phase; put karaya gum in a mortar and grind at 3000rpm, add parecoxib sodium while grinding, and continue grinding for 10 minutes to form a grinding phase. Mix the polyvinyl butyral and the grind, add 0.5 times the weight of ethanol after mixing to form an oil phase, add the dispersed phase to the oil phase, and emulsify with a shearing machine to form an emulsion, at a speed of 2500rpm Stir and spray dry to prepare microspheres; the specific method of emulsification is as follows: the first emulsification speed is 1500 rpm, and the time is 2 minutes; the second emulsification speed is 4500 rpm, and the time is 3 minutes; the third emulsificati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com